Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA
- PMID: 17194753
- PMCID: PMC1820477
- DOI: 10.1128/MCB.01974-06
Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA
Abstract
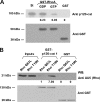
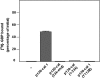
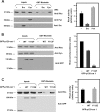
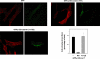
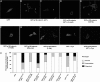
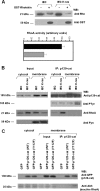
p120-catenin is an adherens junction-associated protein that controls E-cadherin function and stability. p120-catenin also binds intracellular proteins, such as the small GTPase RhoA. In this paper, we identify the p120-catenin N-terminal regulatory domain as the docking site for RhoA. Moreover, we demonstrate that the binding of RhoA to p120-catenin is tightly controlled by the Src family-dependent phosphorylation of p120-catenin on tyrosine residues. The phosphorylation induced by Src and Fyn tyrosine kinases on p120-catenin induces opposite effects on RhoA binding. Fyn, by phosphorylating a residue located in the regulatory domain of p120-catenin (Tyr112), inhibits the interaction of this protein with RhoA. By contrast, the phosphorylation of Tyr217 and Tyr228 by Src promotes a better affinity of p120-catenin towards RhoA. In agreement with these biochemical data, results obtained in cell lines support the important role of these phosphorylation sites in the regulation of RhoA activity by p120-catenin. Taken together, these observations uncover a new regulatory mechanism acting on p120-catenin that contributes to the fine-tuned regulation of the RhoA pathways during specific signaling events.
Figures



Similar articles
-
p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction.Mol Cell Biol. 2003 Apr;23(7):2287-97. doi: 10.1128/MCB.23.7.2287-2297.2003. Mol Cell Biol. 2003. PMID: 12640114 Free PMC article.
-
Inhibition of RhoA by p120 catenin.Nat Cell Biol. 2000 Sep;2(9):637-44. doi: 10.1038/35023588. Nat Cell Biol. 2000. PMID: 10980705
-
Gα(12) binds to the N-terminal regulatory domain of p120(ctn), and downregulates p120(ctn) tyrosine phosphorylation induced by Src family kinases via a RhoA independent mechanism.Exp Cell Res. 2011 Feb 1;317(3):293-306. doi: 10.1016/j.yexcr.2010.10.017. Epub 2010 Oct 23. Exp Cell Res. 2011. PMID: 20974127
-
Emerging roles for p120-catenin in cell adhesion and cancer.Oncogene. 2004 Oct 18;23(48):7947-56. doi: 10.1038/sj.onc.1208161. Oncogene. 2004. PMID: 15489912 Review.
-
p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue.Biochim Biophys Acta. 2007 Jan;1773(1):47-58. doi: 10.1016/j.bbamcr.2006.06.001. Epub 2006 Jun 17. Biochim Biophys Acta. 2007. PMID: 16904204 Review.
Cited by
-
Cigarette smoke extract-induced p120-mediated NF-κB activation in human epithelial cells is dependent on the RhoA/ROCK pathway.Sci Rep. 2016 Sep 2;6:23131. doi: 10.1038/srep23131. Sci Rep. 2016. PMID: 27586697 Free PMC article.
-
Upon Wnt stimulation, Rac1 activation requires Rac1 and Vav2 binding to p120-catenin.J Cell Sci. 2012 Nov 15;125(Pt 22):5288-301. doi: 10.1242/jcs.101030. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946057 Free PMC article.
-
The Cross-Talk Between EGFR and E-Cadherin.Front Cell Dev Biol. 2022 Jan 20;9:828673. doi: 10.3389/fcell.2021.828673. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35127732 Free PMC article. Review.
-
M-Ras/Shoc2 signaling modulates E-cadherin turnover and cell-cell adhesion during collective cell migration.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3536-3545. doi: 10.1073/pnas.1805919116. Epub 2019 Feb 11. Proc Natl Acad Sci U S A. 2019. PMID: 30808747 Free PMC article.
-
N-cadherin/p120 catenin association at cell-cell contacts occurs in cholesterol-rich membrane domains and is required for RhoA activation and myogenesis.J Biol Chem. 2009 Aug 21;284(34):23137-45. doi: 10.1074/jbc.M109.017665. Epub 2009 Jun 22. J Biol Chem. 2009. PMID: 19546217 Free PMC article.
References
-
- Anastasiadis, P. Z., S. Y. Moon, M. A. Thoreson, D. J. Mariner, H. C. Crawford, Y. Zheng, and A. B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637-644. - PubMed
-
- Bryant, D. M., and J. L. Stow. 2004. The ins and outs of E-cadherin trafficking. Trends Cell. Biol. 14:427-434. - PubMed
-
- Castaño, J., I. Raurell, J. Piedra, S. Miravet, M. Duñach, and A. García de Herreros. 2002. β-Catenin N-and C-tails modulate the coordinated binding of adherens junction proteins to β-catenin. J. Biol. Chem. 277:31541-31550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
