Intragenic suppressor mutations restore GTPase and translation functions of a eukaryotic initiation factor 5B switch II mutant
- PMID: 17189426
- PMCID: PMC1820465
- DOI: 10.1128/MCB.01258-06
Intragenic suppressor mutations restore GTPase and translation functions of a eukaryotic initiation factor 5B switch II mutant
Abstract
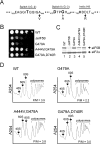
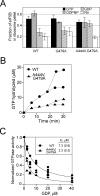
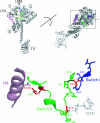
Structural studies of GTP-binding proteins identified the Switch I and Switch II elements as contacting the gamma-phosphate of GTP and undergoing marked conformational changes upon GTP versus GDP binding. Movement of a universally conserved Gly at the N terminus of Switch II is thought to trigger the structural rearrangement of this element. Consistently, we found that mutation of this Gly in the Switch II element of the eukaryotic translation initiation factor 5B (eIF5B) from Saccharomyces cerevisiae impaired cell growth and the guanine nucleotide-binding, GTPase, and ribosomal subunit joining activities of eIF5B. In a screen for mutations that bypassed the critical requirement for this Switch II Gly in eIF5B, intragenic suppressors were identified in the Switch I element and at a residue in domain II of eIF5B that interacts with Switch II. The intragenic suppressors restored yeast cell growth and eIF5B nucleotide-binding, GTP hydrolysis, and subunit joining activities. We propose that the Switch II mutation distorts the geometry of the GTP-binding active site, impairing nucleotide binding and the eIF5B domain movements associated with GTP binding. Accordingly, the Switch I and domain II suppressor mutations induce Switch II to adopt a conformation favorable for nucleotide binding and hydrolysis and thereby reestablish coupling between GTP binding and eIF5B domain movements.
Figures

Similar articles
-
Coupled release of eukaryotic translation initiation factors 5B and 1A from 80S ribosomes following subunit joining.Mol Cell Biol. 2007 Mar;27(6):2384-97. doi: 10.1128/MCB.02254-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242201 Free PMC article.
-
Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining.J Biol Chem. 2006 Mar 31;281(13):8469-75. doi: 10.1074/jbc.M600210200. Epub 2006 Feb 3. J Biol Chem. 2006. PMID: 16461768
-
Uncoupling of initiation factor eIF5B/IF2 GTPase and translational activities by mutations that lower ribosome affinity.Cell. 2002 Dec 27;111(7):1015-25. doi: 10.1016/s0092-8674(02)01171-6. Cell. 2002. PMID: 12507428
-
Universal translation initiation factor IF2/eIF5B.Cold Spring Harb Symp Quant Biol. 2001;66:417-24. doi: 10.1101/sqb.2001.66.417. Cold Spring Harb Symp Quant Biol. 2001. PMID: 12762044 Review. No abstract available.
-
The guanine nucleotide-binding switch in three dimensions.Science. 2001 Nov 9;294(5545):1299-304. doi: 10.1126/science.1062023. Science. 2001. PMID: 11701921 Review.
Cited by
-
Expression, purification and ligand binding properties of the recombinant translation initiation factor (PeIF5B) from Pisum sativum.Mol Cell Biochem. 2010 Nov;344(1-2):33-41. doi: 10.1007/s11010-010-0526-2. Epub 2010 Oct 2. Mol Cell Biochem. 2010. PMID: 20890638
-
Structural integrity of {alpha}-helix H12 in translation initiation factor eIF5B is critical for 80S complex stability.RNA. 2011 Apr;17(4):687-96. doi: 10.1261/rna.2412511. Epub 2011 Feb 18. RNA. 2011. PMID: 21335519 Free PMC article.
-
Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae.Genetics. 2016 May;203(1):65-107. doi: 10.1534/genetics.115.186221. Genetics. 2016. PMID: 27183566 Free PMC article. Review.
-
eIF5B employs a novel domain release mechanism to catalyze ribosomal subunit joining.EMBO J. 2014 May 16;33(10):1177-91. doi: 10.1002/embj.201387344. Epub 2014 Mar 31. EMBO J. 2014. PMID: 24686316 Free PMC article.
-
eIF5A promotes translation of polyproline motifs.Mol Cell. 2013 Jul 11;51(1):35-45. doi: 10.1016/j.molcel.2013.04.021. Epub 2013 May 30. Mol Cell. 2013. PMID: 23727016 Free PMC article.
References
-
- Algire, M. A., D. Maag, and J. R. Lorsch. 2005. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20:251-262. - PubMed
-
- Berghuis, A. M., E. Lee, A. S. Raw, A. G. Gilman, and S. R. Sprang. 1996. Structure of the GDP-Pi complex of Gly203→Ala gialpha1: a mimic of the ternary product complex of galpha-catalyzed GTP hydrolysis. Structure 4:1277-1290. - PubMed
-
- Chakrabarti, P. P., Y. Suveyzdis, A. Wittinghofer, and K. Gerwert. 2004. Fourier transform infrared spectroscopy on the Rap. RapGAP reaction, GTPase activation without an arginine finger. J. Biol. Chem. 279:46226-46233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
