Connecdenn, a novel DENN domain-containing protein of neuronal clathrin-coated vesicles functioning in synaptic vesicle endocytosis
- PMID: 17182770
- PMCID: PMC6674997
- DOI: 10.1523/JNEUROSCI.4608-06.2006
Connecdenn, a novel DENN domain-containing protein of neuronal clathrin-coated vesicles functioning in synaptic vesicle endocytosis
Abstract
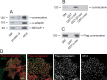
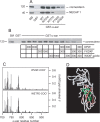
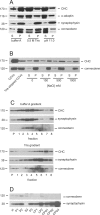
Clathrin-coated vesicles (CCVs) are responsible for the endocytosis of multiple cargo, including synaptic vesicle membranes. We now describe a new CCV protein, termed connecdenn, that contains an N-terminal DENN (differentially expressed in neoplastic versus normal cells) domain, a poorly characterized protein module found in multiple proteins of unrelated function and a C-terminal peptide motif domain harboring three distinct motifs for binding the alpha-ear of the clathrin adaptor protein 2 (AP-2). Connecdenn coimmunoprecipitates and partially colocalizes with AP-2, and nuclear magnetic resonance and peptide competition studies reveal that all three alpha-ear-binding motifs contribute to AP-2 interactions. In addition, connecdenn contains multiple Src homology 3 (SH3) domain-binding motifs and coimmunoprecipitates with the synaptic SH3 domain proteins intersectin and endophilin A1. Interestingly, connecdenn is enriched on neuronal CCVs and is present in the presynaptic compartment of neurons. Moreover, connecdenn has a uniquely stable association with CCV membranes because it resists extraction with Tris and high-salt buffers, unlike most other CCV proteins, but it is not detected on purified synaptic vesicles. Together, these observations suggest that connecdenn functions on the endocytic limb of the synaptic vesicle cycle. Accordingly, disruption of connecdenn interactions with its binding partners through overexpression of the C-terminal peptide motif domain or knock down of connecdenn through lentiviral delivery of small hairpin RNA both lead to defects in synaptic vesicle endocytosis in cultured hippocampal neurons. Thus, we identified connecdenn as a component of the endocytic machinery functioning in synaptic vesicle endocytosis, providing the first evidence of a role for a DENN domain-containing protein in endocytosis.
Figures



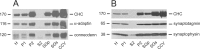
Similar articles
-
Phosphorylation-dependent Regulation of Connecdenn/DENND1 Guanine Nucleotide Exchange Factors.J Biol Chem. 2015 Jul 17;290(29):17999-18008. doi: 10.1074/jbc.M115.636712. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055712 Free PMC article.
-
The connecdenn family, Rab35 guanine nucleotide exchange factors interfacing with the clathrin machinery.J Biol Chem. 2010 Apr 2;285(14):10627-37. doi: 10.1074/jbc.M109.050930. Epub 2010 Feb 12. J Biol Chem. 2010. PMID: 20154091 Free PMC article.
-
The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes.Mol Cell. 2010 Feb 12;37(3):370-82. doi: 10.1016/j.molcel.2009.12.037. Mol Cell. 2010. PMID: 20159556 Free PMC article.
-
Synaptic vesicle endocytosis.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a005645. doi: 10.1101/cshperspect.a005645. Cold Spring Harb Perspect Biol. 2012. PMID: 22763746 Free PMC article. Review.
-
Clathrin-mediated endocytosis at the synaptic terminal: bridging the gap between physiology and molecules.Traffic. 2010 Dec;11(12):1489-97. doi: 10.1111/j.1600-0854.2010.01104.x. Traffic. 2010. PMID: 20633242 Free PMC article. Review.
Cited by
-
Activity-Dependent Degradation of Synaptic Vesicle Proteins Requires Rab35 and the ESCRT Pathway.J Neurosci. 2016 Aug 17;36(33):8668-86. doi: 10.1523/JNEUROSCI.0725-16.2016. J Neurosci. 2016. PMID: 27535913 Free PMC article.
-
Phosphorylation-dependent Regulation of Connecdenn/DENND1 Guanine Nucleotide Exchange Factors.J Biol Chem. 2015 Jul 17;290(29):17999-18008. doi: 10.1074/jbc.M115.636712. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055712 Free PMC article.
-
Genetic alterations within the DENND1A gene in patients with polycystic ovary syndrome (PCOS).PLoS One. 2013 Sep 27;8(9):e77186. doi: 10.1371/journal.pone.0077186. eCollection 2013. PLoS One. 2013. PMID: 24086769 Free PMC article.
-
RabGEFs are a major determinant for specific Rab membrane targeting.J Cell Biol. 2013 Feb 4;200(3):287-300. doi: 10.1083/jcb.201209113. J Cell Biol. 2013. PMID: 23382462 Free PMC article.
-
Intersectin regulates dendritic spine development and somatodendritic endocytosis but not synaptic vesicle recycling in hippocampal neurons.J Biol Chem. 2009 May 1;284(18):12410-9. doi: 10.1074/jbc.M809746200. Epub 2009 Mar 3. J Biol Chem. 2009. PMID: 19258322 Free PMC article.
References
-
- Barik S. Site-directed mutagenesis by double polymerase chain reaction. Mol Biotechnol. 1995;3:1–7. - PubMed
-
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci USA. 2004;101:3833–3838. - PMC - PubMed
-
- Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol. 2006;18:395–406. - PubMed
-
- Burman JL, Wasiak S, Ritter B, de Heuvel E, McPherson PS. Aftiphilin is a component of the clathrin machinery in neurons. FEBS Lett. 2005;579:2177–2184. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
