Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist
- PMID: 17182678
- PMCID: PMC1865977
- DOI: 10.1128/JVI.01634-06
Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist
Abstract
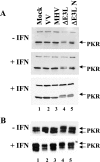
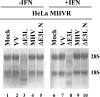
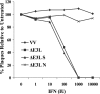
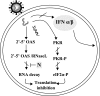
The recent emergence of several new coronaviruses, including the etiological cause of severe acute respiratory syndrome, has significantly increased the importance of understanding virus-host cell interactions of this virus family. We used mouse hepatitis virus (MHV) A59 as a model to gain insight into how coronaviruses affect the type I alpha/beta interferon (IFN) system. We demonstrate that MHV is resistant to type I IFN. Protein kinase R (PKR) and the alpha subunit of eukaryotic translation initiation factor are not phosphorylated in infected cells. The RNase L activity associated with 2',5'-oligoadenylate synthetase is not activated or is blocked, since cellular RNA is not degraded. These results are consistent with lack of protein translation shutoff early following infection. We used a well-established recombinant vaccinia virus (VV)-based expression system that lacks the viral IFN antagonist E3L to screen viral genes for their ability to rescue the IFN sensitivity of the mutant. The nucleocapsid (N) gene rescued VVDeltaE3L from IFN sensitivity. N gene expression prevents cellular RNA degradation and partially rescues the dramatic translation shutoff characteristic of the VVDeltaE3L virus. However, it does not prevent PKR phosphorylation. The results indicate that the MHV N protein is a type I IFN antagonist that likely plays a role in circumventing the innate immune response.
Figures




Similar articles
-
Roles of vaccinia virus genes E3L and K3L and host genes PKR and RNase L during intratracheal infection of C57BL/6 mice.J Virol. 2011 Jan;85(1):550-67. doi: 10.1128/JVI.00254-10. Epub 2010 Oct 13. J Virol. 2011. PMID: 20943971 Free PMC article.
-
Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A.J Virol. 2001 Jun;75(11):5090-8. doi: 10.1128/JVI.75.11.5090-5098.2001. J Virol. 2001. PMID: 11333890 Free PMC article.
-
Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus.J Virol. 2002 May;76(10):4912-8. doi: 10.1128/jvi.76.10.4912-4918.2002. J Virol. 2002. PMID: 11967308 Free PMC article.
-
The interferon system and vaccinia virus evasion mechanisms.J Interferon Cytokine Res. 2009 Sep;29(9):581-98. doi: 10.1089/jir.2009.0073. J Interferon Cytokine Res. 2009. PMID: 19708815 Review.
-
Translational control in virus-infected cells: models for cellular stress responses.Semin Cell Dev Biol. 2005 Feb;16(1):13-20. doi: 10.1016/j.semcdb.2004.11.011. Epub 2004 Dec 30. Semin Cell Dev Biol. 2005. PMID: 15659335 Review.
Cited by
-
Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK.Viruses. 2024 Jan 18;16(1):140. doi: 10.3390/v16010140. Viruses. 2024. PMID: 38257840 Free PMC article.
-
Coronavirus takeover of host cell translation and intracellular antiviral response: a molecular perspective.EMBO J. 2024 Jan;43(2):151-167. doi: 10.1038/s44318-023-00019-8. Epub 2024 Jan 10. EMBO J. 2024. PMID: 38200146 Free PMC article. Review.
-
The Nucleocapsid Proteins of SARS-CoV-2 and Its Close Relative Bat Coronavirus RaTG13 Are Capable of Inhibiting PKR- and RNase L-Mediated Antiviral Pathways.Microbiol Spectr. 2023 Jun 15;11(3):e0099423. doi: 10.1128/spectrum.00994-23. Epub 2023 May 8. Microbiol Spectr. 2023. PMID: 37154717 Free PMC article.
-
Porcine epidemic diarrhea virus strain FJzz1 infection induces type I/III IFNs production through RLRs and TLRs-mediated signaling.Front Immunol. 2022 Jul 25;13:984448. doi: 10.3389/fimmu.2022.984448. eCollection 2022. Front Immunol. 2022. PMID: 35958569 Free PMC article.
-
Antagonism of Protein Kinase R by Large DNA Viruses.Pathogens. 2022 Jul 12;11(7):790. doi: 10.3390/pathogens11070790. Pathogens. 2022. PMID: 35890034 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

