Genetic analysis of Mint/X11 proteins: essential presynaptic functions of a neuronal adaptor protein family
- PMID: 17167098
- PMCID: PMC6674967
- DOI: 10.1523/JNEUROSCI.2855-06.2006
Genetic analysis of Mint/X11 proteins: essential presynaptic functions of a neuronal adaptor protein family
Abstract
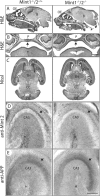
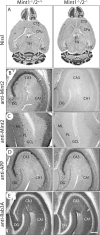
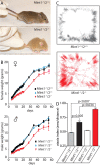
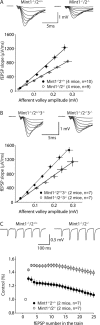
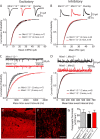
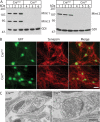
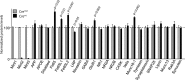
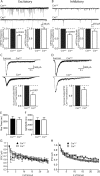
Mints/X11s are adaptor proteins composed of three isoforms: neuron-specific Mints 1 and 2, and the ubiquitously expressed Mint 3. We have now analyzed constitutive and conditional knock-out mice for all three Mints/X11s. We found that approximately 80% of mice lacking both neuron-specific Mint isoforms (Mints 1 and 2) die at birth, whereas mice lacking any other combination of Mint isoforms survive normally. The approximately 20% surviving Mint 1/2 double knock-out mice exhibit a decrease in weight and deficits in motor behaviors. Hippocampal slice electrophysiology uncovered a decline in spontaneous neurotransmitter release, lowered synaptic strength, and enhanced paired-pulse facilitation in Mint-deficient mice, suggesting a decreased presynaptic release probability. Acute ablation of Mint expression in cultured neurons from conditional Mint 1/2/3 triple knock-in mice also revealed a decline in spontaneous release, confirming that deletion of Mints impair presynaptic function. Quantitation of synaptic proteins showed that acute deletion of Mints caused a selective increase in Munc18-1 and Fe65 proteins, and overexpression of Munc18-1 in wild-type neurons also produced a decrease in spontaneous release, suggesting that the interaction of Mints with Munc18-1 may contribute to the presynaptic phenotype observed in Mint-deficient mice. Our studies thus indicate that Mints are important regulators of presynaptic neurotransmitter release that are essential for mouse survival.
Figures



Similar articles
-
A role for Mints in transmitter release: Mint 1 knockout mice exhibit impaired GABAergic synaptic transmission.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1409-14. doi: 10.1073/pnas.252774899. Epub 2003 Jan 23. Proc Natl Acad Sci U S A. 2003. PMID: 12547917 Free PMC article.
-
Regulation of APP-dependent transcription complexes by Mint/X11s: differential functions of Mint isoforms.J Neurosci. 2002 Sep 1;22(17):7340-51. doi: 10.1523/JNEUROSCI.22-17-07340.2002. J Neurosci. 2002. PMID: 12196555 Free PMC article.
-
Mint 3: a ubiquitous mint isoform that does not bind to munc18-1 or -2.Eur J Cell Biol. 1998 Nov;77(3):161-5. doi: 10.1016/S0171-9335(98)80103-9. Eur J Cell Biol. 1998. PMID: 9860131
-
Synaptic transmission regulated by a presynaptic MALS/Liprin-alpha protein complex.Curr Opin Cell Biol. 2006 Apr;18(2):223-7. doi: 10.1016/j.ceb.2006.02.010. Epub 2006 Feb 28. Curr Opin Cell Biol. 2006. PMID: 16504495 Review.
-
[Impaired cognitive function and presynaptic protein complexin in patients with schizophrenia].Seishin Shinkeigaku Zasshi. 2006;108(12):1330-4. Seishin Shinkeigaku Zasshi. 2006. PMID: 17396388 Review. Japanese. No abstract available.
Cited by
-
The SNARE Machinery in Mast Cell Secretion.Front Immunol. 2012 Jun 5;3:143. doi: 10.3389/fimmu.2012.00143. eCollection 2012. Front Immunol. 2012. PMID: 22679448 Free PMC article.
-
Mint3 depletion restricts tumor malignancy of pancreatic cancer cells by decreasing SKP2 expression via HIF-1.Oncogene. 2020 Sep;39(39):6218-6230. doi: 10.1038/s41388-020-01423-8. Epub 2020 Aug 21. Oncogene. 2020. PMID: 32826949 Free PMC article.
-
The effects of amyloid precursor protein on postsynaptic composition and activity.J Biol Chem. 2009 Mar 27;284(13):8495-506. doi: 10.1074/jbc.M900141200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164281 Free PMC article.
-
Synapse and Active Zone Assembly in the Absence of Presynaptic Ca2+ Channels and Ca2+ Entry.Neuron. 2020 Aug 19;107(4):667-683.e9. doi: 10.1016/j.neuron.2020.05.032. Epub 2020 Jun 16. Neuron. 2020. PMID: 32616470 Free PMC article.
-
Conditional deletion of Notch1 and Notch2 genes in excitatory neurons of postnatal forebrain does not cause neurodegeneration or reduction of Notch mRNAs and proteins.J Biol Chem. 2012 Jun 8;287(24):20356-68. doi: 10.1074/jbc.M112.349738. Epub 2012 Apr 13. J Biol Chem. 2012. PMID: 22505716 Free PMC article.
References
-
- Biederer T, Südhof TC. Mints as adaptors: direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275:39803–39806. - PubMed
-
- Borg JP, Straight SW, Kaech SM, de Taddeo-Borg M, Kroon DE, Karnak D, Turner RS, Kim SK, Margolis B. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J Biol Chem. 1998;273:31633–31636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
