Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species
- PMID: 17167081
- PMCID: PMC6674962
- DOI: 10.1523/JNEUROSCI.2531-06.2006
Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species
Abstract
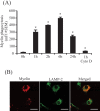
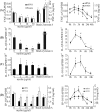
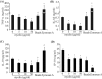
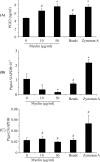
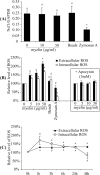
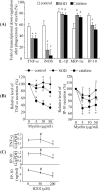
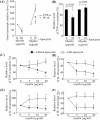
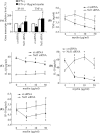
Multiple sclerosis (MS) is pathologically characterized by inflammatory demyelination and neuronal injury. Although phagocytosis of myelin debris by microglia and macrophages in acute MS lesions is well documented, its pathophysiological significance is unclear. Using real-time quantitative PCR, flow cytometry, ELISA, and reactive oxygen species (ROS) measurement assays, we demonstrated that phagocytosis of myelin modulates activation of microglial cells prestimulated by interferon-gamma (IFN-gamma) or a combination of IFN-gamma and lipopolysaccharide with a biphasic temporal pattern, i.e., enhanced production of proinflammatory mediators during the first phase (< or = 6 h), followed by suppression during the second (6-24 h) phase. In this second phase, myelin phagocytosis leads to an enhanced release of prostaglandin E2 and ROS in microglia, whereas the production of anti-inflammatory cytokines (particularly interleukin-10) remains unchanged. Suppression of inflammatory microglial activation by myelin phagocytosis was reversed by treatment with superoxide dismutase and catalase, by inhibition of the NADPH-oxidase complex, or by specific knockdown of the NADPH-oxidase-required adaptor p47-phagocyte oxidase (PHOX). Furthermore, we observed that myelin phagocytosis destabilized tumor necrosis factor-alpha and interferon-induced protein-10 mRNA through an adenine-uridine-rich elements-involved mechanism, which was reversed by blocking the function of NADPH-oxidase complex. We conclude that phagocytosis of myelin suppresses microglial inflammatory activities via enhancement of p47-PHOX-mediated ROS generation. These results suggest that intervention in ROS generation could represent a novel therapeutic strategy to reduce neuroinflammation in MS.
Figures
Similar articles
-
Reactive oxygen species are required for the phagocytosis of myelin by macrophages.J Neuroimmunol. 1998 Dec 1;92(1-2):67-75. doi: 10.1016/s0165-5728(98)00175-1. J Neuroimmunol. 1998. PMID: 9916881
-
AT2R Activation Prevents Microglia Pro-inflammatory Activation in a NOX-Dependent Manner: Inhibition of PKC Activation and p47phox Phosphorylation by PP2A.Mol Neurobiol. 2019 Apr;56(4):3005-3023. doi: 10.1007/s12035-018-1272-9. Epub 2018 Aug 3. Mol Neurobiol. 2019. PMID: 30076526
-
Complex molecular and functional outcomes of single versus sequential cytokine stimulation of rat microglia.J Neuroinflammation. 2016 Mar 24;13(1):66. doi: 10.1186/s12974-016-0531-9. J Neuroinflammation. 2016. PMID: 27009332 Free PMC article.
-
NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair?Free Radic Biol Med. 2014 Nov;76:34-46. doi: 10.1016/j.freeradbiomed.2014.07.033. Epub 2014 Aug 1. Free Radic Biol Med. 2014. PMID: 25091898 Free PMC article. Review.
-
NADPH oxidase as a therapeutic target in Alzheimer's disease.BMC Neurosci. 2008 Dec 3;9 Suppl 2(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. BMC Neurosci. 2008. PMID: 19090996 Free PMC article. Review.
Cited by
-
Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity.Front Immunol. 2022 Aug 29;13:945485. doi: 10.3389/fimmu.2022.945485. eCollection 2022. Front Immunol. 2022. PMID: 36105813 Free PMC article.
-
Polarization of macrophages and microglia in inflammatory demyelination.Neurosci Bull. 2013 Apr;29(2):189-98. doi: 10.1007/s12264-013-1324-0. Epub 2013 Apr 5. Neurosci Bull. 2013. PMID: 23558588 Free PMC article. Review.
-
Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases.Mol Neurobiol. 2016 Mar;53(2):1181-1194. doi: 10.1007/s12035-014-9070-5. Epub 2015 Jan 20. Mol Neurobiol. 2016. PMID: 25598354 Review.
-
Role of oxidant scavengers in the prevention of Ca²+ homeostasis disorders.Molecules. 2010 Oct 15;15(10):7167-87. doi: 10.3390/molecules15107167. Molecules. 2010. PMID: 20953160 Free PMC article. Review.
-
NADPH oxidase in brain injury and neurodegenerative disorders.Mol Neurodegener. 2017 Jan 17;12(1):7. doi: 10.1186/s13024-017-0150-7. Mol Neurodegener. 2017. PMID: 28095923 Free PMC article. Review.
References
-
- Akiyama N, Shimma N, Takashiro Y, Hatori Y, Hirabayashi T, Horie S, Saito T, Murayama T. Decrease in cytosolic phospholipase A2alpha mRNA levels by reactive oxygen species via MAP kinase pathways in PC12 cells: effects of dopaminergic neurotoxins. Cell Signal. 2005;17:597–604. - PubMed
-
- Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–375. - PubMed
-
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. - PubMed
-
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

