Epstein-Barr virus nuclear protein EBNA3C is required for cell cycle progression and growth maintenance of lymphoblastoid cells
- PMID: 17159137
- PMCID: PMC1748255
- DOI: 10.1073/pnas.0604919104
Epstein-Barr virus nuclear protein EBNA3C is required for cell cycle progression and growth maintenance of lymphoblastoid cells
Abstract
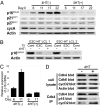
Epstein-Barr virus (EBV) infection converts primary human B cells into continuously proliferating lymphoblastoid cell lines (LCLs). To examine the role of EBV nuclear antigen (EBNA) 3C in the proliferation of LCLs, we established LCLs infected with an EBV recombinant that expresses EBNA3C with a C-terminal fusion to a 4-hydroxytamoxifen (4HT)-dependent mutant estrogen receptor, E3C-HT. In the presence of 4HT, LCLs expressed the E3C-HT protein and grew like WT LCLs. When E3C-HT EBV-infected LCLs were transferred to medium without 4HT, E3C-HT protein slowly disappeared, and the LCLs gradually ceased growing. WT EBNA3C expression from an oriP plasmid transfected into E3C-HT LCLs protected the LCLs from growth arrest in medium without 4HT, whereas expression of EBNA3A or EBNA3B did not. The expression of other EBNA proteins and of LMP1, CD21, CD23, and c-myc was unaffected by EBNA3C inactivation. However, EBNA3C inactivation resulted in the accumulation of p16INK4A, a decrease in the hyperphosphorylated form of the retinoblastoma protein, and a decrease in the proportion of cells in S or G2/M phase. These results indicate that EBNA3C has an essential role in cell cycle progression and the growth maintenance of LCLs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
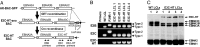



Similar articles
-
Epstein-Barr Virus nuclear protein EBNA3A is critical for maintaining lymphoblastoid cell line growth.J Virol. 2003 Oct;77(19):10437-47. doi: 10.1128/jvi.77.19.10437-10447.2003. J Virol. 2003. PMID: 12970429 Free PMC article.
-
Induction of p16(INK4a) is the major barrier to proliferation when Epstein-Barr virus (EBV) transforms primary B cells into lymphoblastoid cell lines.PLoS Pathog. 2013 Feb;9(2):e1003187. doi: 10.1371/journal.ppat.1003187. Epub 2013 Feb 21. PLoS Pathog. 2013. PMID: 23436997 Free PMC article.
-
Epstein-Barr Virus Nuclear Antigen 3 (EBNA3) Proteins Regulate EBNA2 Binding to Distinct RBPJ Genomic Sites.J Virol. 2015 Dec 30;90(6):2906-19. doi: 10.1128/JVI.02737-15. J Virol. 2015. PMID: 26719268 Free PMC article.
-
The Epstein Barr nuclear antigen EBNA3C regulates transcription, cell transformation and cell migration.Front Biosci. 2002 Mar 1;7:d704-16. doi: 10.2741/subraman. Front Biosci. 2002. PMID: 11861219 Review.
-
The Cooperative Functions of the EBNA3 Proteins Are Central to EBV Persistence and Latency.Pathogens. 2018 Mar 17;7(1):31. doi: 10.3390/pathogens7010031. Pathogens. 2018. PMID: 29562595 Free PMC article. Review.
Cited by
-
Epstein-Barr virus latent genes.Exp Mol Med. 2015 Jan 23;47(1):e131. doi: 10.1038/emm.2014.84. Exp Mol Med. 2015. PMID: 25613728 Free PMC article. Review.
-
Functional modulation of the metastatic suppressor Nm23-H1 by oncogenic viruses.FEBS Lett. 2011 Oct 20;585(20):3174-84. doi: 10.1016/j.febslet.2011.08.007. Epub 2011 Aug 11. FEBS Lett. 2011. PMID: 21846466 Free PMC article. Review.
-
Epstein-Barr virus nuclear antigen 3A protein regulates CDKN2B transcription via interaction with MIZ-1.Nucleic Acids Res. 2014 Sep;42(15):9700-16. doi: 10.1093/nar/gku697. Epub 2014 Aug 4. Nucleic Acids Res. 2014. PMID: 25092922 Free PMC article.
-
Regulation of the metastasis suppressor Nm23-H1 by tumor viruses.Naunyn Schmiedebergs Arch Pharmacol. 2015 Feb;388(2):207-24. doi: 10.1007/s00210-014-1043-8. Epub 2014 Sep 10. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25199839 Free PMC article. Review.
-
Epigenetic Impact on EBV Associated B-Cell Lymphomagenesis.Biomolecules. 2016 Nov 24;6(4):46. doi: 10.3390/biom6040046. Biomolecules. 2016. PMID: 27886133 Free PMC article. Review.
References
-
- Rickinson AB, Kieff E. In: Fields Virology. Knipe DM, Howley PM, editors. Vol 2. Philadelphia: Lippincott; 2001. pp. 2575–2628.
-
- Henle W, Diehl V, Kohn G, zur Hausen H, Henle G. Science. 1967;157:1064–1065. - PubMed
-
- Kieff E, Rickinson AB. In: Fields Virology. Knipe DM, Howley PM, editors. Vol 2. Philadelphia: Lippincott; 2001. pp. 2511–2574.
-
- Shimizu N, Yamaki M, Sakuma S, Ono Y, Takada K. Int J Cancer. 1988;41:744–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

