Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth
- PMID: 17158937
- PMCID: PMC1803144
- DOI: 10.1128/AAC.01510-06
Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth
Abstract
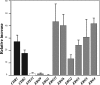
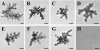
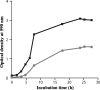
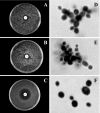
Little information is available about the molecular mechanisms responsible for polyene resistance in pathogenic yeasts. A clinical isolate of Candida glabrata with a poor susceptibility to polyenes, as determined by disk diffusion method and confirmed by determination of MIC, was recovered from a patient treated with amphotericin B. Quantitative analysis of sterols revealed a lack of ergosterol and an accumulation of late sterol intermediates, suggesting a defect in the final steps of the ergosterol pathway. Sequencing of CgERG11, CgERG6, CgERG5, and CgERG4 genes revealed exclusively a unique missense mutation in CgERG6 leading to the substitution of a cysteine by a phenylalanine in the corresponding protein. In addition, real-time reverse transcription-PCR demonstrated an overexpression of genes encoding enzymes involved in late steps of the ergosterol pathway. Moreover, this isolate exhibited a pseudohyphal growth whatever the culture medium used, and ultrastructural changes of the cell wall of blastoconidia were seen consisting in a thinner inner layer. Cell wall alterations were also suggested by the higher susceptibility of growing cells to Calcofluor white. Additionally, complementation of this isolate with a wild-type copy of the CgERG6 gene restored susceptibility to polyenes and a classical morphology. Together, these results demonstrated that mutation in the CgERG6 gene may lead to a reduced susceptibility to polyenes and to a pseudohyphal growth due to the subsequent changes in sterol content of the plasma membrane.
Figures



Similar articles
-
A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata.Antimicrob Agents Chemother. 2008 Oct;52(10):3701-9. doi: 10.1128/AAC.00423-08. Epub 2008 Aug 11. Antimicrob Agents Chemother. 2008. PMID: 18694952 Free PMC article.
-
Erg6p is essential for antifungal drug resistance, plasma membrane properties and cell wall integrity in Candida glabrata.FEMS Yeast Res. 2022 Sep 24;21(1):foac045. doi: 10.1093/femsyr/foac045. FEMS Yeast Res. 2022. PMID: 36047961
-
Genome-wide expression profiling reveals genes associated with amphotericin B and fluconazole resistance in experimentally induced antifungal resistant isolates of Candida albicans.J Antimicrob Chemother. 2004 Aug;54(2):376-85. doi: 10.1093/jac/dkh336. Epub 2004 Jun 16. J Antimicrob Chemother. 2004. PMID: 15201233
-
Antifungal drug resistance mechanisms.Expert Rev Anti Infect Ther. 2009 May;7(4):453-60. doi: 10.1586/eri.09.18. Expert Rev Anti Infect Ther. 2009. PMID: 19400764 Review.
-
Metabolism of sterols in yeast.CRC Crit Rev Microbiol. 1978;6(4):301-41. doi: 10.3109/10408417809090625. CRC Crit Rev Microbiol. 1978. PMID: 365459 Review. No abstract available.
Cited by
-
Molecular mechanisms of resistance to 5-fluorocytosine in laboratory mutants of Candida glabrata.Mycopathologia. 2011 Jan;171(1):11-21. doi: 10.1007/s11046-010-9342-1. Epub 2010 Jul 9. Mycopathologia. 2011. PMID: 20617462
-
Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance.Nat Commun. 2016 Mar 29;7:11128. doi: 10.1038/ncomms11128. Nat Commun. 2016. PMID: 27020939 Free PMC article.
-
Linking Cellular Morphogenesis with Antifungal Treatment and Susceptibility in Candida Pathogens.J Fungi (Basel). 2019 Feb 21;5(1):17. doi: 10.3390/jof5010017. J Fungi (Basel). 2019. PMID: 30795580 Free PMC article. Review.
-
Antifungal resistance and new strategies to control fungal infections.Int J Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. Epub 2011 Dec 1. Int J Microbiol. 2012. PMID: 22187560 Free PMC article.
-
One small step for a yeast--microevolution within macrophages renders Candida glabrata hypervirulent due to a single point mutation.PLoS Pathog. 2014 Oct 30;10(10):e1004478. doi: 10.1371/journal.ppat.1004478. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25356907 Free PMC article.
References
-
- Arthington, B. A., L. G. Bennett, P. L. Skatrud, C. J. Guynn, R. J. Barbuch, C. E. Ulbright, and M. Bard. 1991. Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene 102:39-44. - PubMed
-
- Ashman, W. H., R. J. Barbuch, C. E. Ulbright, H. W. Jarrett, and M. Bard. 1991. Cloning and disruption of the yeast C-8 sterol isomerase gene. Lipids 26:628-632. - PubMed
-
- Barker, K. S., S. Crisp, N. Wiederhold, R. E. Lewis, B. Bareither, J. Eckstein, R. Barbuch, M. Bard, and P. D. Rogers. 2004. Genome-wide expression profiling reveals genes associated with amphotericin B and fluconazole resistance in experimentally induced antifungal resistant isolates of Candida albicans. J. Antimicrob. Chemother. 54:376-385. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

