Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1)
- PMID: 17154366
- PMCID: PMC3412056
- DOI: 10.1002/jcp.20904
Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1)
Abstract
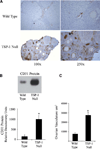
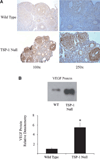
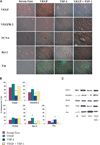
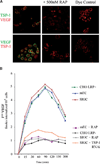
VEGF is a potent pro-angiogenic factor whose effects are opposed by a host of anti-angiogenic proteins, including thrombospondin-1 (TSP-1). We have previously shown that VEGF has important extravascular roles in the ovary and that VEGF and TSP-1 are inversely expressed throughout the ovarian cycle. To date, however, a causal interaction between TSP-1 and VEGF has not been identified. Here, we show that TSP-1 has a direct inhibitory effect on VEGF by binding the growth factor and internalizing it via LRP-1. Mice lacking TSP-1 are subfertile and exhibited ovarian hypervascularization and altered ovarian morphology. Treatment of ovarian cells with TSP-1 decreased VEGF levels and rendered the cells more susceptible to TNFalpha-induced apoptosis. Knockdown of TSP-1, through RNA interference, resulted in overexpression of VEGF and reduced cytokine-induced apoptosis. In conclusion, we demonstrate a direct inhibitory effect of TSP-1 on VEGF in the ovary. TSP-1's regulation of VEGF appears to be an important mediator of ovarian angiogenesis and follicle development.
Copyright 2006 Wiley-Liss, Inc.
Figures

Similar articles
-
Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development.Biol Reprod. 2005 May;72(5):1071-8. doi: 10.1095/biolreprod.104.031120. Epub 2004 Dec 22. Biol Reprod. 2005. PMID: 15616224
-
Thrombospondin-1 expression is increased during follicular atresia in the primate ovary.Endocrinology. 2008 Jan;149(1):185-92. doi: 10.1210/en.2007-0835. Epub 2007 Sep 20. Endocrinology. 2008. PMID: 17884943
-
Thrombospondin-1 inhibits angiogenesis and promotes follicular atresia in a novel in vitro angiogenesis assay.Endocrinology. 2010 Mar;151(3):1280-9. doi: 10.1210/en.2009-0686. Epub 2010 Jan 15. Endocrinology. 2010. PMID: 20080874
-
[Role of thrombospondin-1 in the development of kidney diseases].Med Sci (Paris). 2013 Dec;29(12):1131-7. doi: 10.1051/medsci/20132912017. Epub 2013 Dec 20. Med Sci (Paris). 2013. PMID: 24356144 Review. French.
-
Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2.Cold Spring Harb Perspect Med. 2012 May;2(5):a006627. doi: 10.1101/cshperspect.a006627. Cold Spring Harb Perspect Med. 2012. PMID: 22553494 Free PMC article. Review.
Cited by
-
Thrombospondin-1: A Key Protein That Induces Fibrosis in Diabetic Complications.J Diabetes Res. 2020 Jun 11;2020:8043135. doi: 10.1155/2020/8043135. eCollection 2020. J Diabetes Res. 2020. PMID: 32626782 Free PMC article. Review.
-
The thrombospondins.Cold Spring Harb Perspect Biol. 2011 Oct 1;3(10):a009712. doi: 10.1101/cshperspect.a009712. Cold Spring Harb Perspect Biol. 2011. PMID: 21875984 Free PMC article. Review.
-
Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level.FASEB J. 2009 Oct;23(10):3368-76. doi: 10.1096/fj.09-131649. Epub 2009 Jun 15. FASEB J. 2009. PMID: 19528255 Free PMC article.
-
Expression of thrombospondin-1 and Ski are prognostic factors in advanced gastric cancer.Int J Clin Oncol. 2011 Apr;16(2):145-52. doi: 10.1007/s10147-010-0147-5. Epub 2010 Nov 25. Int J Clin Oncol. 2011. PMID: 21107877
-
The Contributions of Thrombospondin-1 to Epilepsy Formation.Neurosci Bull. 2024 May;40(5):658-672. doi: 10.1007/s12264-024-01194-2. Epub 2024 Mar 26. Neurosci Bull. 2024. PMID: 38528256 Review.
References
-
- Amis SJ, Coulter-Smith SD, Crow JC, Maclean AB, Perrett CW. Micro-vessel quantification in benign and malignant ovarian tumors. Int J Gynecol Cancer. 2005;15:58–65. - PubMed
-
- Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. - PubMed
-
- Barboni B, Turriani M, Galeati G, Spinaci M, Bacci ML, Forni M, Mattioli M. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod. 2000;63:858–864. - PubMed
-
- Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275:32164–32173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

