Sirtuins: a conserved key unlocking AceCS activity
- PMID: 17141505
- PMCID: PMC2396790
- DOI: 10.1016/j.tibs.2006.11.002
Sirtuins: a conserved key unlocking AceCS activity
Abstract
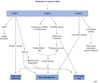
Bacterial acetyl-coenzyme A (acetyl-CoA) synthetase (AceCS), an evolutionarily conserved enzyme that converts acetate to acetyl-CoA, is activated by sirtuin-mediated deacetylation. Two recent studies show that this mechanism of regulation is also crucial for mammalian AceCS activity, indicating that control of metabolism at the step of converting acetate to acetyl-CoA is conserved. These findings highlight a metabolic regulatory network controlled by sirtuins that has implications for the mechanisms of calorie restriction and modulation of mammalian lifespan.
Figures

Similar articles
-
Conserved metabolic regulatory functions of sirtuins.Cell Metab. 2008 Feb;7(2):104-12. doi: 10.1016/j.cmet.2007.11.006. Cell Metab. 2008. PMID: 18249170 Review.
-
Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10224-10229. doi: 10.1073/pnas.0603968103. Epub 2006 Jun 20. Proc Natl Acad Sci U S A. 2006. PMID: 16788062 Free PMC article.
-
Acetate metabolism and aging: An emerging connection.Mech Ageing Dev. 2010 Jul-Aug;131(7-8):511-6. doi: 10.1016/j.mad.2010.05.001. Epub 2010 May 15. Mech Ageing Dev. 2010. PMID: 20478325
-
Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10230-10235. doi: 10.1073/pnas.0604392103. Epub 2006 Jun 21. Proc Natl Acad Sci U S A. 2006. PMID: 16790548 Free PMC article.
-
SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism.Cold Spring Harb Symp Quant Biol. 2011;76:267-77. doi: 10.1101/sqb.2011.76.010850. Epub 2011 Nov 23. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22114326 Review.
Cited by
-
Role of SIRT3 in neurological diseases and rehabilitation training.Metab Brain Dis. 2023 Jan;38(1):69-89. doi: 10.1007/s11011-022-01111-4. Epub 2022 Nov 14. Metab Brain Dis. 2023. PMID: 36374406 Free PMC article. Review.
-
Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle.Aging (Albany NY). 2009 Aug 15;1(9):771-83. doi: 10.18632/aging.100075. Aging (Albany NY). 2009. PMID: 20157566 Free PMC article.
-
Pma1, a P-type proton ATPase, is a determinant of chronological life span in fission yeast.J Biol Chem. 2010 Nov 5;285(45):34616-20. doi: 10.1074/jbc.M110.175562. Epub 2010 Sep 9. J Biol Chem. 2010. PMID: 20829365 Free PMC article.
-
SIRT1, miR-132 and miR-212 link human longevity to Alzheimer's Disease.Sci Rep. 2018 May 31;8(1):8465. doi: 10.1038/s41598-018-26547-6. Sci Rep. 2018. PMID: 29855513 Free PMC article.
-
Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism.J Biol Chem. 2009 Jan 2;284(1):158-164. doi: 10.1074/jbc.M807976200. Epub 2008 Nov 11. J Biol Chem. 2009. PMID: 19001417 Free PMC article.
References
-
- Guarente L, Picard F. Calorie restriction – the SIR2 connection. Cell. 2005;120:473–482. - PubMed
-
- Lamming DW, et al. Small molecules that regulate lifespan: evidence for xenohormesis. Mol. Microbiol. 2004;53:1003–1009. - PubMed
-
- Dhahbi JM, et al. Calories and aging alter gene expression for gluconeogenic, glycolytic, and nitrogen-metabolizing enzymes. Am. J. Physiol. 1999;277:E352–E360. - PubMed
-
- Hagopian K, et al. Krebs cycle enzymes from livers of old mice are differentially regulated by caloric restriction. Exp. Gerontol. 2004;39:1145–1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG019972-03/AG/NIA NIH HHS/United States
- R01 AG019719-05/AG/NIA NIH HHS/United States
- P01 AG027916-010003/AG/NIA NIH HHS/United States
- R01 AG019972-04/AG/NIA NIH HHS/United States
- R01 AG019972-05/AG/NIA NIH HHS/United States
- R01 GM068072/GM/NIGMS NIH HHS/United States
- P01 AG027916/AG/NIA NIH HHS/United States
- T32 AG023480-05/AG/NIA NIH HHS/United States
- R01 AG019972-01/AG/NIA NIH HHS/United States
- R01 AG028730/AG/NIA NIH HHS/United States
- R01 AG019972/AG/NIA NIH HHS/United States
- T32 AG023480/AG/NIA NIH HHS/United States
- R01 AG028730-01A1/AG/NIA NIH HHS/United States
- R01 AG019719-04/AG/NIA NIH HHS/United States
- R01 GM068072-01/GM/NIGMS NIH HHS/United States
- R01 AG019972-02/AG/NIA NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

