An essential role for FGF receptor signaling in lens development
- PMID: 17116415
- PMCID: PMC1931569
- DOI: 10.1016/j.semcdb.2006.10.002
An essential role for FGF receptor signaling in lens development
Abstract
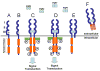
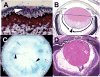
Since the days of Hans Spemann, the ocular lens has served as one of the most important developmental systems for elucidating fundamental processes of induction and differentiation. More recently, studies in the lens have contributed significantly to our understanding of cell cycle regulation and apoptosis. Over 20 years of accumulated evidence using several different vertebrate species has suggested that fibroblast growth factors (FGFs) and/or fibroblast growth factor receptors (FGFRs) play a key role in lens development. FGFR signaling has been implicated in lens induction, lens cell proliferation and survival, lens fiber differentiation and lens regeneration. Here we will review and discuss historical and recent evidence suggesting that (FGFR) signaling plays a vital and universal role in multiple aspects of lens development.
Figures

Similar articles
-
Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation.Dev Dyn. 2005 Jun;233(2):516-27. doi: 10.1002/dvdy.20356. Dev Dyn. 2005. PMID: 15778993
-
Multiple roles of Equarin during lens development.Dev Growth Differ. 2014 Apr;56(3):199-205. doi: 10.1111/dgd.12121. Epub 2014 Feb 20. Dev Growth Differ. 2014. PMID: 24673466
-
Sef is a negative regulator of fiber cell differentiation in the ocular lens.Differentiation. 2010 Jul;80(1):53-67. doi: 10.1016/j.diff.2010.05.005. Epub 2010 Jun 9. Differentiation. 2010. PMID: 20542628 Free PMC article.
-
FGF/FGFR signaling in bone formation: progress and perspectives.Growth Factors. 2012 Apr;30(2):117-23. doi: 10.3109/08977194.2012.656761. Epub 2012 Feb 1. Growth Factors. 2012. PMID: 22292523 Review.
-
Cell cycle regulation in the developing lens.Semin Cell Dev Biol. 2006 Dec;17(6):686-97. doi: 10.1016/j.semcdb.2006.10.004. Epub 2006 Nov 1. Semin Cell Dev Biol. 2006. PMID: 17218126 Free PMC article. Review.
Cited by
-
Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis.Cells. 2022 Aug 30;11(17):2708. doi: 10.3390/cells11172708. Cells. 2022. PMID: 36078116 Free PMC article.
-
Comparison of FGFR1 expression on lens epithelial cells between adults and fetuses.Int J Ophthalmol. 2011;4(1):37-9. doi: 10.3980/j.issn.2222-3959.2011.01.08. Epub 2011 Feb 18. Int J Ophthalmol. 2011. PMID: 22553605 Free PMC article.
-
Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression.Development. 2016 Jan 15;143(2):318-28. doi: 10.1242/dev.127860. Epub 2015 Dec 10. Development. 2016. PMID: 26657765 Free PMC article.
-
Analysis of long-range chromatin contacts, compartments and looping between mouse embryonic stem cells, lens epithelium and lens fibers.Epigenetics Chromatin. 2024 Apr 20;17(1):10. doi: 10.1186/s13072-024-00533-x. Epigenetics Chromatin. 2024. PMID: 38643244 Free PMC article.
-
Patterns of gene expression in microarrays and expressed sequence tags from normal and cataractous lenses.Hum Genomics. 2012 Sep 1;6(1):14. doi: 10.1186/1479-7364-6-14. Hum Genomics. 2012. PMID: 23244575 Free PMC article.
References
-
- Kuszak JR, Costello MJ. The Structure of the Vertebrate Lens. In: Lovicu FJ, Robinson ML, editors. The Structure of the Vertebrate Lens. New York, NY: Cambridge University Press; 2004. pp. 71–118.
-
- Duncan MK, Cvekl A, Kantorow M, Piatigorsky J. Lens Crystallins. In: Lovicu FJ, Robinson ML, editors. Lens Crystallins. New York, NY: Cambridge University Press; 2004. pp. 119–150.
-
- Lovicu FJ, Robinson ML, editors. 2004. Cambridge University Press; New York, NY: Development of the Ocular Lens.
-
- Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3608–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

