Proteins in load-bearing junctions: the histidine-rich metal-binding protein of mussel byssus
- PMID: 17115717
- PMCID: PMC1892233
- DOI: 10.1021/bi061677n
Proteins in load-bearing junctions: the histidine-rich metal-binding protein of mussel byssus
Abstract
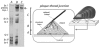
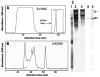
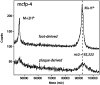
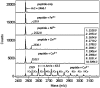
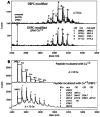
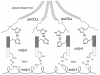
Building complex load-bearing scaffolds depends on effective ways of joining functionally different biomacromolecules. The junction between collagen fibers and foamlike adhesive plaques in mussel byssus is robust despite the strikingly dissimilar connected structures. mcfp-4, the matrix protein from this junction, and its presecreted form from the foot tissue of Mytilus californianus were isolated and characterized. mcfp-4 has a mass of approximately 93 kDa as determined by MALDI-TOF mass spectrometry. Its composition is dominated by histidine (22 mol %), but levels of lysine, arginine, and aspartate are also significant. A small amount of 3,4-dihydroxyphenyl-l-alanine (2 mol %) can be detected by amino acid analysis and redox cycling assays. The cDNA-deduced sequence of mcfp-4 reveals multiple variants with highly repetitive internal structures, including approximately 36 tandemly repeated His-rich decapeptides (e.g., HVHTHRVLHK) in the N-terminal half and 16 somewhat more degenerate aspartate-rich undecapeptides (e.g., DDHVNDIAQTA) in the C-terminal half. Incubation of a synthetic peptide based on the His-rich decapeptide with Fe3+, Co2+, Ni2+, Zn2+, and Cu2+ indicates that only Cu is strongly bound. MALDI-TOF mass spectrometry of the peptide modified with diethyl pyrocarbonate before and after Cu binding suggests that histidine residues dominate Cu binding. In contrast, the aspartate-rich undecapeptides preferentially bind Ca2+. mcfp-4 is strategically positioned to function as a macromolecular bifunctional linker by using metal ions to couple its own His-rich domains to the His-rich termini of the preCOLs. Ca2+ may mediate coupling of the C-terminus to other calcium-binding plaque proteins.
Figures



Similar articles
-
Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus.J Biol Chem. 2006 Sep 8;281(36):26150-8. doi: 10.1074/jbc.M604357200. Epub 2006 Jul 14. J Biol Chem. 2006. PMID: 16844688
-
Coating proteins: structure and cross-linking in fp-1 from the green shell mussel Perna canaliculus.Biochemistry. 2005 Dec 6;44(48):15915-23. doi: 10.1021/bi051530g. Biochemistry. 2005. PMID: 16313194 Free PMC article.
-
Probing the adhesive footprints of Mytilus californianus byssus.J Biol Chem. 2006 Apr 21;281(16):11090-6. doi: 10.1074/jbc.M510792200. Epub 2006 Feb 22. J Biol Chem. 2006. PMID: 16495227
-
Metal protein interactions.Prog Food Nutr Sci. 1987;11(3-4):363-400. Prog Food Nutr Sci. 1987. PMID: 3328221 Review.
-
The peculiar collagens of mussel byssus.Matrix Biol. 1998 Jun;17(2):93-106. doi: 10.1016/s0945-053x(98)90023-3. Matrix Biol. 1998. PMID: 9694590 Review.
Cited by
-
Protein-Based Biological Materials: Molecular Design and Artificial Production.Chem Rev. 2023 Mar 8;123(5):2049-2111. doi: 10.1021/acs.chemrev.2c00621. Epub 2023 Jan 24. Chem Rev. 2023. PMID: 36692900 Free PMC article. Review.
-
Secretion of byssal threads in Mytilus galloprovincialis: quantitative and qualitative values after spawning stress.J Comp Physiol B. 2010 Jan;180(1):95-104. doi: 10.1007/s00360-009-0392-y. Epub 2009 Jul 18. J Comp Physiol B. 2010. PMID: 19618191
-
Hypoxia weakens mussel attachment by interrupting DOPA cross-linking during adhesive plaque curing.J R Soc Interface. 2018 Oct 24;15(147):20180489. doi: 10.1098/rsif.2018.0489. J R Soc Interface. 2018. PMID: 30355807 Free PMC article.
-
Cross-linking by protein oxidation in the rapidly setting gel-based glues of slugs.J Exp Biol. 2011 May 15;214(Pt 10):1699-706. doi: 10.1242/jeb.051581. J Exp Biol. 2011. PMID: 21525316 Free PMC article.
-
Mass Spectrometry-Based Protein Footprinting for Higher-Order Structure Analysis: Fundamentals and Applications.Chem Rev. 2020 May 27;120(10):4355-4454. doi: 10.1021/acs.chemrev.9b00815. Epub 2020 Apr 22. Chem Rev. 2020. PMID: 32319757 Free PMC article. Review.
References
-
- Currey JD. Bones: Structure and Mechanics. Princeton University Press; Princeton, NJ.: 2002.
-
- Linke WA, Ivemeyer M, Olivieri N, Kolmerer B, Rüegg JC, Labeit S. Towards a molecular understanding of the elasticity of titin. J. Mol. Biol. 1996;261:62–71. - PubMed
-
- Hasler EM, Herzog W, Wu JZ, Müller W, Wyss U. Articular cartilage biomechanics: Theoretical models material properties and biosynthetic response. Crit. Rev. Biomed. Eng. 1999;27:415–488. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

