p63 regulates proliferation and differentiation of developmentally mature keratinocytes
- PMID: 17114587
- PMCID: PMC1635152
- DOI: 10.1101/gad.1463206
p63 regulates proliferation and differentiation of developmentally mature keratinocytes
Abstract
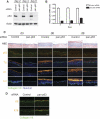
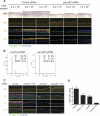
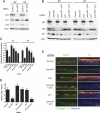
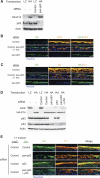
p63 is a multi-isoform p53 family member required for epidermal development. Contrasting roles for p63 in either the initial commitment to the stratified epithelial cell fate or in stem cell-based self-renewal have been proposed. To investigate p63 function in a post-developmental context, we used siRNAs directed against p63 to down-regulate p63 expression in regenerating human epidermis. Loss of p63 resulted in severe tissue hypoplasia and inhibited both stratification and differentiation in a cell-autonomous manner. Although p63-deficient cells exhibited hypoproliferation, differentiation defects were not due to tissue hypoplasia. Simultaneous p63 and p53 knockdown rescued the cell proliferation defect of p63 knockdown alone but failed to restore differentiation, suggesting that defects in epidermal proliferation and differentiation are mediated via p53-dependent and -independent mechanisms, respectively. Furthermore, DeltaNp63 isoforms are the main mediators of p63 effects, although TAp63 isoforms may contribute to late differentiation. These data indicate that p63 is required for both the proliferative and differentiation potential of developmentally mature keratinocytes.
Figures
Similar articles
-
DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells.Cancer Res. 2010 May 15;70(10):4034-44. doi: 10.1158/0008-5472.CAN-09-4063. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442293
-
TAp63-dependent induction of growth differentiation factor 15 (GDF15) plays a critical role in the regulation of keratinocyte differentiation.Oncogene. 2008 Jan 17;27(4):409-20. doi: 10.1038/sj.onc.1210658. Epub 2007 Jul 16. Oncogene. 2008. PMID: 17637746
-
miR-203 represses 'stemness' by repressing DeltaNp63.Cell Death Differ. 2008 Jul;15(7):1187-95. doi: 10.1038/cdd.2008.69. Epub 2008 May 16. Cell Death Differ. 2008. PMID: 18483491
-
Control of keratinocyte proliferation and differentiation by p63.Cell Cycle. 2007 Feb 1;6(3):295-9. doi: 10.4161/cc.6.3.3753. Epub 2007 Feb 28. Cell Cycle. 2007. PMID: 17264679 Review.
-
Dynamic life of a skin keratinocyte: an intimate tryst with the master regulator p63.Indian J Exp Biol. 2011 Oct;49(10):721-31. Indian J Exp Biol. 2011. PMID: 22013738 Review.
Cited by
-
Epidermal stem cells in orthopaedic regenerative medicine.Int J Mol Sci. 2013 May 31;14(6):11626-42. doi: 10.3390/ijms140611626. Int J Mol Sci. 2013. PMID: 23727934 Free PMC article. Review.
-
Pirh2 E3 ubiquitin ligase modulates keratinocyte differentiation through p63.J Invest Dermatol. 2013 May;133(5):1178-87. doi: 10.1038/jid.2012.466. Epub 2012 Dec 13. J Invest Dermatol. 2013. PMID: 23235527 Free PMC article.
-
FOXM1 regulates proliferation, senescence and oxidative stress in keratinocytes and cancer cells.Aging (Albany NY). 2016 Jul;8(7):1384-97. doi: 10.18632/aging.100988. Aging (Albany NY). 2016. PMID: 27385468 Free PMC article.
-
Different expression patterns of p63 and p73 in Felis catus papillomavirus type 2-associated feline Merkel cell carcinomas and other epidermal carcinomas.J Vet Med Sci. 2024 Jan 10;86(1):39-48. doi: 10.1292/jvms.23-0293. Epub 2023 Nov 28. J Vet Med Sci. 2024. PMID: 38030281 Free PMC article.
-
Pathway Regulation of p63, a Director of Epithelial Cell Fate.Front Endocrinol (Lausanne). 2015 Apr 28;6:51. doi: 10.3389/fendo.2015.00051. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25972840 Free PMC article. Review.
References
-
- Bond, J., Haughton, M., Blaydes, J., Gire, V., Wynford-Thomas, D., Wyllie, F. Evidence that transcriptional activation by p53 plays a direct role in the induction of cellular senescence. Oncogene. 1996;13:2097–2104. - PubMed
-
- Candi, E., Rufini, A., Terrinoni, A., Dinsdale, D., Ranalli, M., Paradisi, A., De Laurenzi, V., Spagnoli, L.G., Catani, M.V., Ramadan, S., et al. Differential roles of p63 isoforms in epidermal development: Selective genetic complementation in p63 null mice. Cell Death Differ. 2006;13:1037–1047. - PubMed
-
- Carroll, D.K., Carroll, J.S., Leong, C.O., Cheng, F., Brown, M., Mills, A.A., Brugge, J.S., Ellisen, L.W. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 2006;8:551–561. - PubMed
-
- Delehedde, M., Cho, S.H., Hamm, R., Brisbay, S., Ananthaswamy, H.N., Kripke, M., McDonnell, T.J. Impact of Bcl-2 and Ha-ras on keratinocytes in organotypic culture. J. Invest. Dermatol. 2001;116:366–373. - PubMed
-
- Donehower, L.A., Harvey, M., Slagle, B.L., McArthur, M.J., Montgomery, C.A., Jr., Butel, J.S., Bradley, A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
