Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells
- PMID: 17108032
- PMCID: PMC1797494
- DOI: 10.1128/JVI.01740-06
Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells
Abstract
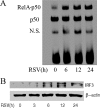
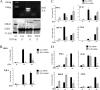
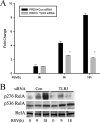
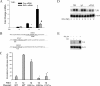
Respiratory syncytial virus (RSV) is one of the most common viral pathogens causing severe lower respiratory tract infections in infants and young children. Infected host cells detect and respond to RNA viruses using different mechanisms in a cell-type-specific manner, including retinoic acid-inducible gene I (RIG-I)-dependent and Toll-like receptor (TLR)-dependent pathways. Because the relative contributions of these two pathways in the recognition of RSV infection are unknown, we examined their roles in this study. We found that RIG-I helicase binds RSV transcripts within 12 h of infection. Short interfering RNA (siRNA)-mediated RIG-I "knockdown" significantly inhibited early nuclear factor-kappaB (NF-kappaB) and interferon response factor 3 (IRF3) activation 9 h postinfection (p.i.). Consistent with this finding, RSV-induced beta interferon (IFN-beta), interferon-inducible protein 10 (IP-10), chemokine ligand 5 (CCL-5), and IFN-stimulated gene 15 (ISG15) expression levels were decreased in RIG-I-silenced cells during the early phase of infection but not at later times (18 h p.i.). In contrast, siRNA-mediated TLR3 knockdown did not affect RSV-induced NF-kappaB binding but did inhibit IFN-beta, IP-10, CCL-5, and ISG15 expression at late times of infection. Further studies revealed that TLR3 knockdown significantly reduced NF-kappaB/RelA transcription by its ability to block the activating phosphorylation of NF-kappaB/RelA at serine residue 276. We further found that TLR3 induction following RSV infection was regulated by RIG-I-dependent IFN-beta secreted from infected airway epithelial cells and was mediated by both IFN response-stimulated element (ISRE) and signal transducer and activator of transcription (STAT) sites in its proximal promoter. Together these findings indicate distinct temporal roles of RIG-I and TLR3 in mediating RSV-induced innate immune responses, which are coupled to distinct pathways controlling NF-kappaB activation.
Figures


Similar articles
-
Respiratory Syncytial Virus Infection Upregulates NLRC5 and Major Histocompatibility Complex Class I Expression through RIG-I Induction in Airway Epithelial Cells.J Virol. 2015 Aug;89(15):7636-45. doi: 10.1128/JVI.00349-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972545 Free PMC article.
-
BRD4 Couples NF-κB/RelA with Airway Inflammation and the IRF-RIG-I Amplification Loop in Respiratory Syncytial Virus Infection.J Virol. 2017 Feb 28;91(6):e00007-17. doi: 10.1128/JVI.00007-17. Print 2017 Mar 15. J Virol. 2017. PMID: 28077651 Free PMC article.
-
Ataxia telangiectasia mutated kinase mediates NF-κB serine 276 phosphorylation and interferon expression via the IRF7-RIG-I amplification loop in paramyxovirus infection.J Virol. 2015 Mar;89(5):2628-42. doi: 10.1128/JVI.02458-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520509 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
RSV Reprograms the CDK9•BRD4 Chromatin Remodeling Complex to Couple Innate Inflammation to Airway Remodeling.Viruses. 2020 Apr 22;12(4):472. doi: 10.3390/v12040472. Viruses. 2020. PMID: 32331282 Free PMC article. Review.
Cited by
-
An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25.J Virol. 2022 Oct 12;96(19):e0129722. doi: 10.1128/jvi.01297-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102648 Free PMC article. Review.
-
Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection.J Immunol. 2013 Sep 1;191(5):2526-37. doi: 10.4049/jimmunol.1300477. Epub 2013 Jul 26. J Immunol. 2013. PMID: 23894198 Free PMC article.
-
Respiratory Syncytial Virus Infection Upregulates NLRC5 and Major Histocompatibility Complex Class I Expression through RIG-I Induction in Airway Epithelial Cells.J Virol. 2015 Aug;89(15):7636-45. doi: 10.1128/JVI.00349-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972545 Free PMC article.
-
Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology.Viruses. 2016 May 12;8(5):124. doi: 10.3390/v8050124. Viruses. 2016. PMID: 27187445 Free PMC article. Review.
-
Innate immune recognition of respiratory syncytial virus infection.BMB Rep. 2014 Apr;47(4):184-91. doi: 10.5483/bmbrep.2014.47.4.050. BMB Rep. 2014. PMID: 24568879 Free PMC article. Review.
References
-
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257-263. - PubMed
-
- Brasier, A. R., J. E. Tate, and J. F. Habener. 1989. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. BioTechniques 7:1116-1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

