Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor
- PMID: 17108019
- PMCID: PMC1797529
- DOI: 10.1128/JVI.01702-06
Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor
Abstract
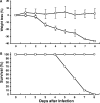
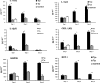
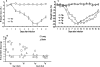
Animal models for severe acute respiratory syndrome (SARS) coronavirus infection of humans are needed to elucidate SARS pathogenesis and develop vaccines and antivirals. We developed transgenic mice expressing human angiotensin-converting enzyme 2, a functional receptor for the virus, under the regulation of a global promoter. A transgenic lineage, designated AC70, was among the best characterized against SARS coronavirus infection, showing weight loss and other clinical manifestations before reaching 100% mortality within 8 days after intranasal infection. High virus titers were detected in the lungs and brains of transgene-positive (Tg+) mice on days 1 and 3 after infection. Inflammatory mediators were also detected in these tissues, coinciding with high levels of virus replication. Lower virus titers were also detected in other tissues, including blood. In contrast, infected transgene-negative (Tg-) mice survived without showing any clinical illness. Pathologic examination suggests that the extensive involvement of the central nervous system likely contributed to the death of Tg+ mice, even though viral pneumonia was present. Preliminary studies with mice of a second lineage, AC63, in which the transgene expression was considerably less abundant than that in the AC70 line, revealed that virus replication was largely restricted to the lungs but not the brain. Importantly, despite significant weight loss, infected Tg+ AC63 mice eventually recovered from the illness without any mortality. The severity of the disease that developed in these transgenic mice--AC70 in particular--makes these mouse models valuable not only for evaluating the efficacy of antivirals and vaccines, but also for studying SARS coronavirus pathogenesis.
Figures





Similar articles
-
Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2.J Virol. 2009 Jun;83(11):5451-65. doi: 10.1128/JVI.02272-08. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297479 Free PMC article.
-
Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus.J Virol. 2007 Jan;81(2):813-21. doi: 10.1128/JVI.02012-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079315 Free PMC article.
-
Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2.J Virol. 2008 Aug;82(15):7264-75. doi: 10.1128/JVI.00737-08. Epub 2008 May 21. J Virol. 2008. PMID: 18495771 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
-
Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus.Cell Mol Life Sci. 2004 Nov;61(21):2738-43. doi: 10.1007/s00018-004-4242-5. Cell Mol Life Sci. 2004. PMID: 15549175 Free PMC article. Review.
Cited by
-
Characterization of Collaborative Cross mouse founder strain CAST/EiJ as a novel model for lethal COVID-19.Sci Rep. 2024 Oct 24;14(1):25147. doi: 10.1038/s41598-024-77087-1. Sci Rep. 2024. PMID: 39448712 Free PMC article.
-
Characterizing neuroinvasion and neuropathology of SARS-CoV-2 by using AC70 human ACE2 transgenic mice.Front Microbiol. 2024 Sep 24;15:1455462. doi: 10.3389/fmicb.2024.1455462. eCollection 2024. Front Microbiol. 2024. PMID: 39380676 Free PMC article.
-
Characterizing a lethal CAG-ACE2 transgenic mouse model for SARS-CoV-2 infection using Cas9-enhanced nanopore sequencing.Transgenic Res. 2024 Sep 25. doi: 10.1007/s11248-024-00413-w. Online ahead of print. Transgenic Res. 2024. PMID: 39320390
-
Characterization of Collaborative Cross mouse founder strain CAST/EiJ as a novel model for lethal COVID-19.Res Sq [Preprint]. 2024 Jul 29:rs.3.rs-4675061. doi: 10.21203/rs.3.rs-4675061/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Oct 24;14(1):25147. doi: 10.1038/s41598-024-77087-1. PMID: 39149485 Free PMC article. Updated. Preprint.
-
Characterization of Unique Pathological Features of COVID-Associated Coagulopathy: Studies with AC70 hACE2 Transgenic Mice Highly Permissive to SARS-CoV-2 Infection.PLoS Pathog. 2024 Jun 24;20(6):e1011777. doi: 10.1371/journal.ppat.1011777. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38913740 Free PMC article.
References
-
- Ding, Y., L. He, Q. Zhang, Z. Huang, X. Che, J. Hou, H. Wang, H. Shen, L. Qiu, Z. Li, J. Geng, J. Cai, H. Han, X. Li, W. Kang, D. Weng, P. Liang, and S. Jiang. 2004. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 203:622-630. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

