Genomic expression profiling of human inflammatory cardiomyopathy (DCMi) suggests novel therapeutic targets
- PMID: 17106732
- PMCID: PMC1820750
- DOI: 10.1007/s00109-006-0122-9
Genomic expression profiling of human inflammatory cardiomyopathy (DCMi) suggests novel therapeutic targets
Abstract
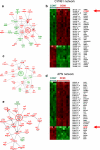
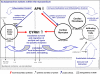
The clinical phenotype of human dilated cardiomyopathy (DCM) encompasses a broad spectrum of etiologically distinct disorders. As targeting of etiology-related pathogenic pathways may be more efficient than current standard heart failure treatment, we obtained the genomic expression profile of a DCM subtype characterized by cardiac inflammation to identify possible new therapeutic targets in humans. In this inflammatory cardiomyopathy (DCMi), a distinctive cardiac expression pattern not described in any previous study of cardiac disorders was observed. Two significantly altered gene networks of particular interest and possible interdependence centered around the cysteine-rich angiogenic inducer 61 (CYR61) and adiponectin (APN) gene. CYR61 overexpression, as in human DCMi hearts in situ, was similarly induced by inflammatory cytokines in vascular endothelial cells in vitro. APN was strongly downregulated in DCMi hearts and completely abolished cytokine-dependent CYR61 induction in vitro. Dysbalance between the CYR61 and APN networks may play a pathogenic role in DCMi and contain novel therapeutic targets. Multiple immune cell-associated genes were also deregulated (e.g., chemokine ligand 14, interleukin-17D, nuclear factors of activated T cells). In contrast to previous investigations in patients with advanced or end-stage DCM where etiology-related pathomechanisms are overwhelmed by unspecific processes, the deregulations detected in this study occurred at a far less severe and most probably fully reversible disease stage.
Figures
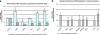
Similar articles
-
Adiponectin expression in patients with inflammatory cardiomyopathy indicates favourable outcome and inflammation control.Eur Heart J. 2011 May;32(9):1134-47. doi: 10.1093/eurheartj/ehq498. Epub 2011 Jan 29. Eur Heart J. 2011. PMID: 21278397
-
Inflammatory dilated cardiomyopathy (DCMI).Herz. 2005 Sep;30(6):535-44. doi: 10.1007/s00059-005-2730-5. Herz. 2005. PMID: 16170686 Review.
-
Nicotinamide phosphoribosyltransferase/pre-B-cell colony enhancing factor/visfatin plasma levels and clinical outcome in patients with dilated cardiomyopathy.J Card Fail. 2015 Apr;21(4):330-8. doi: 10.1016/j.cardfail.2014.12.001. Epub 2014 Dec 9. J Card Fail. 2015. PMID: 25498756
-
Neural cell adhesion molecule expression in dilated cardiomyopathy is associated with intramyocardial inflammation and hypertrophy.Int J Cardiol. 2017 Aug 15;241:322-325. doi: 10.1016/j.ijcard.2017.03.072. Epub 2017 Mar 18. Int J Cardiol. 2017. PMID: 28343767
-
Treatment of inflammatory dilated cardiomyopathy and (peri)myocarditis with immunosuppression and i.v. immunoglobulins.Herz. 2004 Sep;29(6):624-36. doi: 10.1007/s00059-004-2628-7. Herz. 2004. PMID: 15912438 Review.
Cited by
-
Molecular characterization and association analysis of FBXO40 with partial hematological indexes in pig.Mol Biol Rep. 2010 Oct;37(7):3393-400. doi: 10.1007/s11033-009-9928-1. Epub 2009 Nov 27. Mol Biol Rep. 2010. PMID: 19943117
-
Network-based computational approach to identify genetic links between cardiomyopathy and its risk factors.IET Syst Biol. 2020 Apr;14(2):75-84. doi: 10.1049/iet-syb.2019.0074. IET Syst Biol. 2020. PMID: 32196466 Free PMC article.
-
Machine learning-based classification and diagnosis of clinical cardiomyopathies.Physiol Genomics. 2020 Sep 1;52(9):391-400. doi: 10.1152/physiolgenomics.00063.2020. Epub 2020 Aug 3. Physiol Genomics. 2020. PMID: 32744882 Free PMC article.
-
Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure.Cardiovasc Res. 2010 Jun 1;86(3):353-64. doi: 10.1093/cvr/cvq056. Epub 2010 Feb 22. Cardiovasc Res. 2010. PMID: 20176815 Free PMC article. Review.
-
Genomics, transcriptional profiling, and heart failure.J Am Coll Cardiol. 2009 May 12;53(19):1752-9. doi: 10.1016/j.jacc.2008.12.064. J Am Coll Cardiol. 2009. PMID: 19422981 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s00109-005-0664-2', 'is_inner': False, 'url': 'https://doi.org/10.1007/s00109-005-0664-2'}, {'type': 'PubMed', 'value': '15931504', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15931504/'}]}
- Poller W, Kühl U, Tschoepe C, Pauschinger M, Fechner H, Schultheiss H-P (2005) Genome-environment interactions in the molecular pathogenesis of dilated cardiomyopathy. J Mol Med 83:579–586 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/01.CIR.0000155616.07901.35', 'is_inner': False, 'url': 'https://doi.org/10.1161/01.cir.0000155616.07901.35'}, {'type': 'PubMed', 'value': '15699250', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15699250/'}]}
- Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss H-P (2005) High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “Idiopathic” left ventricular dysfunction. Circulation 111:887–893 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/CIRCULATIONAHA.105.548156', 'is_inner': False, 'url': 'https://doi.org/10.1161/circulationaha.105.548156'}, {'type': 'PubMed', 'value': '16172268', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16172268/'}]}
- Kühl U, Pauschinger M, Seeberg B, Noutsias M, Poller W, Schultheiss H-P (2005) Virus persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112:1965–1970 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1161/01.CIR.0000072766.67150.51', 'is_inner': False, 'url': 'https://doi.org/10.1161/01.cir.0000072766.67150.51'}, {'type': 'PubMed', 'value': '12771005', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12771005/'}]}
- Kühl U, Pauschinger M, Schwimmbeck P, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss H-P (2003) Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 107:2793–2798 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1152/physiolgenomics.00259.2004', 'is_inner': False, 'url': 'https://doi.org/10.1152/physiolgenomics.00259.2004'}, {'type': 'PubMed', 'value': '15831843', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15831843/'}]}
- Sanoudou D, Vafiadaki E, Arvanitis DA, Kranias E, Kontrogianni-Konstantopoulos A (2005) Array lessons from the heart: focus on the genome and transcriptome of cardiomyopathies. Physiol Genomics 21:131–143 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

