Cytokine and integrin stimulation synergize to promote higher levels of GATA-2, c-myb, and CD34 protein in primary human hematopoietic progenitors from bone marrow
- PMID: 17095623
- PMCID: PMC1852192
- DOI: 10.1182/blood-2006-05-026039
Cytokine and integrin stimulation synergize to promote higher levels of GATA-2, c-myb, and CD34 protein in primary human hematopoietic progenitors from bone marrow
Abstract
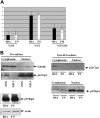
We have previously shown that engagement of the integrins VLA-4 and VLA-5 to the fibronectin fragment CH-296 in combination with cytokines sustained the capacity of cultured human CD34(+) cells to undergo hematopoiesis in immunodeficient mice for 7 to 12 months, whereas this capacity was rapidly lost in cells cultured in suspension with the same cytokines. In the current study, we assessed the molecular pathways that might explain the loss of long-term engraftment capacity in cells cultured in suspension. Although the cell cycle profile was similar between cells cultured in suspension versus on fibronectin, levels of cell death were higher in the suspended cultures. While the CDK inhibitors p27Kip1 and p57Kip2 were present at equal levels in cells from both cultures, low levels of p21Cip1 were detectable only in the cytoplasmic compartment of cells cultured in suspension. Cytoplasmic location of p21Cip1 has been linked to monocytic differentiation. The levels of c-myb and GATA-2, transcription factors associated with stem cell maintenance, were higher in cells cultured on fibronectin as compared with suspension. In contrast, the levels of PU.1, which is induced during myeloid differentiation, were higher in cells cultured in suspension. There were no significant differences in surface expression of CD34 on the cells after culture, but total CD34 protein, assessed by immunoblotting, was significantly higher in cells cultured on fibronectin. Our data suggest that, in the presence of cytokines, the engagement of VLA-4 and VLA-5 integrins to the fibronectin fragment CH-296 preserves the expression of specific transcription factors associated with primitive stem cell maintenance. In contrast, a lack of integrin engagement leads to the induction of cellular markers associated with myeloid differentiation.
Figures



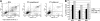
Similar articles
-
High cyclin-dependent kinase inhibitors in Bcl-2 and Bcl-xL-expressing CD34+-proliferating haematopoietic progenitors.Br J Haematol. 2000 Sep;110(3):654-62. doi: 10.1046/j.1365-2141.2000.02227.x. Br J Haematol. 2000. PMID: 10997978
-
Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins.J Exp Med. 1995 May 1;181(5):1805-15. doi: 10.1084/jem.181.5.1805. J Exp Med. 1995. PMID: 7536795 Free PMC article.
-
GATA-2/estrogen receptor chimera regulates cytokine-dependent growth of hematopoietic cells through accumulation of p21(WAF1) and p27(Kip1) proteins.Blood. 2002 Nov 15;100(10):3512-20. doi: 10.1182/blood-2002-04-1177. Epub 2002 Jul 12. Blood. 2002. PMID: 12393444
-
Expression and function of integrins on hematopoietic progenitor cells.Acta Haematol. 1997;97(1-2):13-21. doi: 10.1159/000203655. Acta Haematol. 1997. PMID: 8980606 Review.
-
VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells.Leuk Lymphoma. 1997 Feb;24(5-6):423-35. doi: 10.3109/10428199709055581. Leuk Lymphoma. 1997. PMID: 9086434 Review.
Cited by
-
Fluorophore-conjugated iron oxide nanoparticle labeling and analysis of engrafting human hematopoietic stem cells.Stem Cells. 2008 Feb;26(2):517-24. doi: 10.1634/stemcells.2007-0016. Epub 2007 Nov 29. Stem Cells. 2008. PMID: 18055451 Free PMC article.
-
Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells.Stem Cells. 2008 Aug;26(8):2173-82. doi: 10.1634/stemcells.2007-1104. Epub 2008 May 29. Stem Cells. 2008. PMID: 18511601 Free PMC article.
-
Hematopoietic niche and bone meet.Curr Opin Support Palliat Care. 2008 Sep;2(3):211-7. doi: 10.1097/SPC.0b013e32830d5c12. Curr Opin Support Palliat Care. 2008. PMID: 18685423 Free PMC article. Review.
-
Alternative RNA splicing produces multiple forms of c-Myb with unique transcriptional activities.Mol Cell Biol. 2008 Mar;28(6):2091-101. doi: 10.1128/MCB.01870-07. Epub 2008 Jan 14. Mol Cell Biol. 2008. PMID: 18195038 Free PMC article.
-
Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors.Adv Drug Deliv Rev. 2010 Sep 30;62(12):1167-74. doi: 10.1016/j.addr.2010.09.013. Epub 2010 Oct 13. Adv Drug Deliv Rev. 2010. PMID: 20920540 Free PMC article. Review.
References
-
- Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. - PubMed
-
- Miller JS, McCullar V, Verfaillie CM. Ex vivo culture of CD34+/Lin-/DR-cells in stroma-derived soluble factors, interleukin-3, and macrophage inflammatory protein-1alpha maintains not only myeloid but also lymphoid progenitors in a novel switch culture assay. Blood. 1998;91:4516–4522. - PubMed
-
- Orlic D, Anderson S, Biesecker LG, Sorrentino BP, Bodine DM. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, p45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proc Natl Acad Sci U S A. 1995;92:4601–4605. - PMC - PubMed
-
- Persons DA, Allay JA, Allay ER, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. - PubMed
-
- Melotti P, Calabretta B. Ets-2 and c-Myb act independently in regulating expression of the hematopoietic stem cell antigen CD34. J Biol Chem. 1994;269:25303–25309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

