Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury
- PMID: 17090684
- PMCID: PMC1859960
- DOI: 10.1073/pnas.0602841103
Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury
Abstract
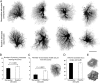
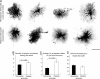
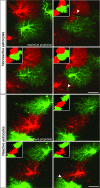
Reactive astrocytes in neurotrauma, stroke, or neurodegeneration are thought to undergo cellular hypertrophy, based on their morphological appearance revealed by immunohistochemical detection of glial fibrillary acidic protein, vimentin, or nestin, all of them forming intermediate filaments, a part of the cytoskeleton. Here, we used a recently established dye-filling method to reveal the full three-dimensional shape of astrocytes assessing the morphology of reactive astrocytes in two neurotrauma models. Both in the denervated hippocampal region and the lesioned cerebral cortex, reactive astrocytes increased the thickness of their main cellular processes but did not extend to occupy a greater volume of tissue than nonreactive astrocytes. Despite this hypertrophy of glial fibrillary acidic protein-containing cellular processes, interdigitation between adjacent hippocampal astrocytes remained minimal. This work helps to redefine the century-old concept of hypertrophy of reactive astrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

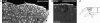
Similar articles
-
Regional difference of reactive astrogliosis following traumatic brain injury revealed by hGFAP-GFP transgenic mice.Neurosci Lett. 2012 Apr 4;513(2):155-9. doi: 10.1016/j.neulet.2012.02.023. Epub 2012 Feb 17. Neurosci Lett. 2012. PMID: 22343312
-
Podoplanin: a marker for reactive gliosis in gliomas and brain injury.J Neuropathol Exp Neurol. 2015 Jan;74(1):64-74. doi: 10.1097/NEN.0000000000000150. J Neuropathol Exp Neurol. 2015. PMID: 25470350
-
A subpopulation of reactive astrocytes at the immediate site of cerebral cortical injury.Exp Neurol. 1997 Jul;146(1):199-205. doi: 10.1006/exnr.1997.6518. Exp Neurol. 1997. PMID: 9225753
-
The role of astrocytes and complement system in neural plasticity.Int Rev Neurobiol. 2007;82:95-111. doi: 10.1016/S0074-7742(07)82005-8. Int Rev Neurobiol. 2007. PMID: 17678957 Review.
-
Role of glial filaments in cells and tumors of glial origin: a review.J Neurosurg. 1997 Sep;87(3):420-30. doi: 10.3171/jns.1997.87.3.0420. J Neurosurg. 1997. PMID: 9285609 Review.
Cited by
-
Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion.Histochem Cell Biol. 2013 Jul;140(1):81-91. doi: 10.1007/s00418-013-1110-0. Epub 2013 Jun 12. Histochem Cell Biol. 2013. PMID: 23756782
-
Contralateral Astrocyte Response to Acute Optic Nerve Damage Is Mitigated by PANX1 Channel Activity.Int J Mol Sci. 2023 Oct 27;24(21):15641. doi: 10.3390/ijms242115641. Int J Mol Sci. 2023. PMID: 37958624 Free PMC article.
-
Morphological plasticity of astroglia: Understanding synaptic microenvironment.Glia. 2015 Dec;63(12):2133-51. doi: 10.1002/glia.22821. Epub 2015 Mar 18. Glia. 2015. PMID: 25782611 Free PMC article. Review.
-
Utilizing human cerebral organoids to model breast cancer brain metastasis in culture.Breast Cancer Res. 2024 Jul 1;26(1):108. doi: 10.1186/s13058-024-01865-y. Breast Cancer Res. 2024. PMID: 38951862 Free PMC article.
-
Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice.FASEB J. 2013 Jan;27(1):187-98. doi: 10.1096/fj.12-208660. Epub 2012 Oct 4. FASEB J. 2013. PMID: 23038755 Free PMC article.
References
-
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Science. 2001;291:657–661. - PubMed
-
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Cell. 2005;120:421–433. - PubMed
-
- Mulligan SJ, MacVicar BA. Nature. 2004;431:195–199. - PubMed
-
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Nat Neurosci. 2003;6:43–50. - PubMed
-
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Nat Neurosci. 1999;2:139–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

