Activation of the poly(ADP-ribose) polymerase pathway in human heart failure
- PMID: 17088946
- PMCID: PMC1626594
- DOI: 10.2119/2006-00043.Molnar
Activation of the poly(ADP-ribose) polymerase pathway in human heart failure
Abstract
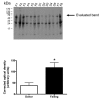
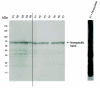
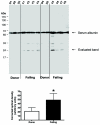
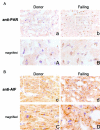
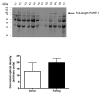
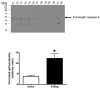
Poly(ADP-ribose) polymerase (PARP) activation has been implicated in the pathogenesis of acute and chronic myocardial dysfunction and heart failure. The goal of the present study was to investigate PARP activation in human heart failure, and to correlate PARP activation with various indices of apoptosis and oxidative and nitrosative stress in healthy (donor) and failing (NYHA class III-IV) human heart tissue samples. Higher levels of oxidized protein end-products were found in failing hearts compared with donor heart samples. On the other hand, no differences in tyrosine nitration (a marker of peroxynitrite generation) were detected. Activation of PARP was demonstrated in the failing hearts by an increased abundance of poly-ADP ribosylated proteins. Immunohistochemical analysis revealed that PARP activation was localized to the nucleus of the cardiomyocytes from the failing hearts. The expression of full-length PARP-1 was not significantly different in donor and failing hearts. The expression of caspase-9, in contrast, was significantly higher in the failing than in the donor hearts. Immunohistochemical analysis was used to detect the activation of mitochondrial apoptotic pathways. We found no significant translocation of apoptosis-inducing factor (AIF) into the nucleus. Overall, the current data provide evidence of oxidative stress and PARP activation in human heart failure. Interventional studies with antioxidants or PARP inhibitors are required to define the specific roles of these factors in the pathogenesis of human heart failure.
Figures
Similar articles
-
Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes.Mol Med. 2006 Sep-Oct;12(9-10):221-8. doi: 10.2119/2006–00055.Toth-Zsamboki. Mol Med. 2006. PMID: 17225870 Free PMC article.
-
Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure.J Pharmacol Exp Ther. 2005 Mar;312(3):891-8. doi: 10.1124/jpet.104.077164. Epub 2004 Nov 2. J Pharmacol Exp Ther. 2005. PMID: 15523000
-
Potential role of poly(adenosine 5'-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock.Crit Care Med. 2006 Apr;34(4):1073-9. doi: 10.1097/01.CCM.0000206470.47721.8D. Crit Care Med. 2006. PMID: 16484919
-
Poly (ADP-ribose) polymerase activation and circulatory shock.Novartis Found Symp. 2007;280:92-103; discussion 103-7, 160-4. doi: 10.1007/0-387-36005-0_16. Novartis Found Symp. 2007. PMID: 17380790 Review.
-
Nitrosative stress and pharmacological modulation of heart failure.Trends Pharmacol Sci. 2005 Jun;26(6):302-10. doi: 10.1016/j.tips.2005.04.003. Trends Pharmacol Sci. 2005. PMID: 15925705 Free PMC article. Review.
Cited by
-
Distinct cardiodynamic and molecular characteristics during early and late stages of sepsis-induced myocardial dysfunction.Life Sci. 2007 Jul 4;81(4):306-16. doi: 10.1016/j.lfs.2007.05.021. Epub 2007 Jun 13. Life Sci. 2007. PMID: 17612571 Free PMC article.
-
Hyperglycemia as a Risk Factor of Ischemic Stroke.J Drug Metab Toxicol. 2013 Jun 29;4(4):153. doi: 10.4172/2157-7609.1000153. J Drug Metab Toxicol. 2013. PMID: 25328819 Free PMC article.
-
Salvage of nicotinamide adenine dinucleotide plays a critical role in the bioenergetic recovery of post-hypoxic cardiomyocytes.Br J Pharmacol. 2015 Oct;172(20):4817-32. doi: 10.1111/bph.13252. Epub 2015 Oct 14. Br J Pharmacol. 2015. PMID: 26218637 Free PMC article.
-
Sp1 Targeted PARP1 Inhibition Protects Cardiomyocytes From Myocardial Ischemia-Reperfusion Injury via Downregulation of Autophagy.Front Cell Dev Biol. 2021 May 25;9:621906. doi: 10.3389/fcell.2021.621906. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34124031 Free PMC article.
-
PARP1 promote autophagy in cardiomyocytes via modulating FoxO3a transcription.Cell Death Dis. 2018 Oct 15;9(11):1047. doi: 10.1038/s41419-018-1108-6. Cell Death Dis. 2018. PMID: 30323296 Free PMC article.
References
-
- Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–40. - PubMed
-
- Ferrari R, Guardigli G, Mele D, Percoco GF, Ceconi C, Curello S. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004;10:1699–711. - PubMed
-
- Szabo C, Liaudet L, Hagl S, Szabo C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004;61:471–80. - PubMed
-
- Tyagi SC, Hayden MR. Role of nitric oxide in matrix remodeling in diabetes and heart failure. Heart Fail Rev. 2003;8:23–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
