Histone acetyltransferase MOZ acts as a co-activator of Nrf2-MafK and induces tumour marker gene expression during hepatocarcinogenesis
- PMID: 17083329
- PMCID: PMC1863558
- DOI: 10.1042/BJ20061194
Histone acetyltransferase MOZ acts as a co-activator of Nrf2-MafK and induces tumour marker gene expression during hepatocarcinogenesis
Abstract
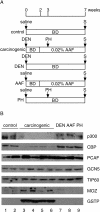
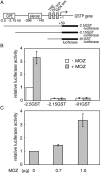
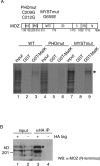
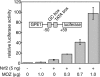
HATs (histone acetyltransferases) contribute to the regulation of gene expression, and loss or dysregulation of these activities may link to tumorigenesis. Here, we demonstrate that expression levels of HATs, p300 and CBP [CREB (cAMP-response-element-binding protein)-binding protein] were decreased during chemical hepatocarcinogenesis, whereas expression of MOZ (monocytic leukaemia zinc-finger protein; MYST3)--a member of the MYST [MOZ, Ybf2/Sas3, Sas2 and TIP60 (Tat-interacting protein, 60 kDa)] acetyltransferase family--was induced. Although the MOZ gene frequently is rearranged in leukaemia, we were unable to detect MOZ rearrangement in livers with hyperplastic nodules. We examined the effect of MOZ on hepatocarcinogenic-specific gene expression. GSTP (glutathione S-transferase placental form) is a Phase II detoxification enzyme and a well-known tumour marker that is specifically elevated during hepatocarcinogenesis. GSTP gene activation is regulated mainly by the GPE1 (GSTP enhancer 1) enhancer element, which is recognized by the Nrf2 (nuclear factor-erythroid 2 p45 subunit-related factor 2)-MafK heterodimer. We found that MOZ enhances GSTP promoter activity through GPE1 and acts as a co-activator of the Nrf2-MafK heterodimer. Further, exogenous MOZ induced GSTP expression in rat hepatoma H4IIE cells. These results suggest that during early hepatocarcinogenesis, aberrantly expressed MOZ may induce GSTP expression through the Nrf2-mediated pathway.
Figures


Similar articles
-
Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis.Biochem J. 2004 Jun 1;380(Pt 2):515-21. doi: 10.1042/BJ20031948. Biochem J. 2004. PMID: 14960151 Free PMC article.
-
Regulation of GST-P gene expression during hepatocarcinogenesis.Methods Enzymol. 2005;401:42-61. doi: 10.1016/S0076-6879(05)01003-7. Methods Enzymol. 2005. PMID: 16399378
-
Regulation of glutathione transferase P: a tumor marker of hepatocarcinogenesis.Biochem Biophys Res Commun. 2007 Jun 8;357(3):575-8. doi: 10.1016/j.bbrc.2007.03.174. Epub 2007 Apr 9. Biochem Biophys Res Commun. 2007. PMID: 17434454 Review.
-
Indigofera suffruticosa Mill extracts up-regulate the expression of the π class of glutathione S-transferase and NAD(P)H: quinone oxidoreductase 1 in rat Clone 9 liver cells.Food Chem Toxicol. 2013 Sep;59:610-7. doi: 10.1016/j.fct.2013.06.042. Epub 2013 Jul 3. Food Chem Toxicol. 2013. PMID: 23831193
-
MOZ fusion proteins in acute myeloid leukaemia.Biochem Soc Symp. 2006;(73):23-39. doi: 10.1042/bss0730023. Biochem Soc Symp. 2006. PMID: 16626284 Review.
Cited by
-
The nuclear factor erythroid 2-related factor 2/p53 axis in breast cancer.Biochem Med (Zagreb). 2023 Oct 15;33(3):030504. doi: 10.11613/BM.2023.030504. Biochem Med (Zagreb). 2023. PMID: 37841775 Free PMC article. Review.
-
Role of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modifications.Mol Biol Cell. 2009 Mar;20(6):1606-17. doi: 10.1091/mbc.e08-07-0762. Epub 2009 Jan 21. Mol Biol Cell. 2009. PMID: 19158375 Free PMC article.
-
Molecular architecture of quartet MOZ/MORF histone acetyltransferase complexes.Mol Cell Biol. 2008 Nov;28(22):6828-43. doi: 10.1128/MCB.01297-08. Epub 2008 Sep 15. Mol Cell Biol. 2008. PMID: 18794358 Free PMC article.
-
Down-regulation of the transcriptional mediator subunit Med1 contributes to the loss of expression of metastasis-associated dapk1 in human cancers and cancer cells.Int J Cancer. 2009 Oct 1;125(7):1566-74. doi: 10.1002/ijc.24493. Int J Cancer. 2009. PMID: 19521987 Free PMC article.
-
SPBP is a sulforaphane induced transcriptional coactivator of NRF2 regulating expression of the autophagy receptor p62/SQSTM1.PLoS One. 2014 Jan 9;9(1):e85262. doi: 10.1371/journal.pone.0085262. eCollection 2014. PLoS One. 2014. PMID: 24416372 Free PMC article.
References
-
- Yang X. J., Ogryzko V. V., Nishikawa J., Howard B. H., Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. - PubMed
-
- Bannister A. J., Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

