Canonical notch signaling functions as a commitment switch in the epidermal lineage
- PMID: 17079689
- PMCID: PMC1620020
- DOI: 10.1101/gad.1477606
Canonical notch signaling functions as a commitment switch in the epidermal lineage
Abstract
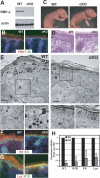
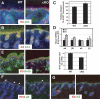
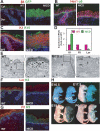
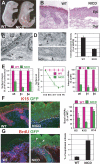
Mammalian epidermis consists of a basal layer of proliferative progenitors that gives rise to multiple differentiating layers to provide a waterproof envelope covering the skin surface. To accomplish this, progenitor cells must detach from the basal layer, move upward, and execute a terminal differentiation program consisting of three distinct stages: spinous, granular layer, and stratum corneum. Notch signaling has been implicated in late stages of differentiation, but the commitment switch remains unknown. Here we show with loss and gain-of-function studies that active Notch intracellular domain (NICD) and its obligate canonical signaling partner RBP-J act at the basal/suprabasal juncture to induce spinous and down-regulate basal fate. Spinous layers are absent in RBP-J conditional null epidermis and expanded when Notch1 signaling is elevated transgenically in epidermis. We show that RBP-J is essential for mediating both spinous gene activation and basal gene repression. In contrast, the NICD/RBP-J target gene Hes1 is expressed in spinous layers and mediates spinous gene induction but not basal gene repression. These data uncover an early role for RBP-J and Notch in commitment of epidermal cells to terminally differentiate and reveal that spinous gene induction is mediated by a Hes1-dependent mechanism, while basal gene repression occurs independently of Hes1.
Figures




Similar articles
-
Multiple roles of Notch signaling in the regulation of epidermal development.Dev Cell. 2008 Apr;14(4):594-604. doi: 10.1016/j.devcel.2008.01.017. Dev Cell. 2008. PMID: 18410734
-
Interaction of Wnt/β-catenin and notch signaling in the early stage of cardiac differentiation of P19CL6 cells.J Cell Biochem. 2012 Feb;113(2):629-39. doi: 10.1002/jcb.23390. J Cell Biochem. 2012. PMID: 21956839
-
RUNX3 directly interacts with intracellular domain of Notch1 and suppresses Notch signaling in hepatocellular carcinoma cells.Exp Cell Res. 2010 Jan 15;316(2):149-57. doi: 10.1016/j.yexcr.2009.09.025. Epub 2009 Oct 2. Exp Cell Res. 2010. PMID: 19800882
-
Role of CSL-dependent and independent Notch signaling pathways in cell apoptosis.Apoptosis. 2016 Jan;21(1):1-12. doi: 10.1007/s10495-015-1188-z. Apoptosis. 2016. PMID: 26496776 Review.
-
Notch signaling and the developing skin epidermis.Adv Exp Med Biol. 2012;727:131-41. doi: 10.1007/978-1-4614-0899-4_10. Adv Exp Med Biol. 2012. PMID: 22399344 Review.
Cited by
-
Ectopic Atoh1 expression drives Merkel cell production in embryonic, postnatal and adult mouse epidermis.Development. 2015 Jul 15;142(14):2533-44. doi: 10.1242/dev.123141. Epub 2015 Jul 2. Development. 2015. PMID: 26138479 Free PMC article.
-
Divergent regulation of functionally distinct γ-tubulin complexes during differentiation.J Cell Biol. 2016 Jun 20;213(6):679-92. doi: 10.1083/jcb.201601099. Epub 2016 Jun 13. J Cell Biol. 2016. PMID: 27298324 Free PMC article.
-
Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling.Dis Model Mech. 2010 Sep-Oct;3(9-10):545-56. doi: 10.1242/dmm.006031. Epub 2010 Aug 10. Dis Model Mech. 2010. PMID: 20699479 Free PMC article. Review.
-
RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus.J Neurosci. 2010 Oct 13;30(41):13794-807. doi: 10.1523/JNEUROSCI.1567-10.2010. J Neurosci. 2010. PMID: 20943920 Free PMC article.
-
JunB defines functional and structural integrity of the epidermo-pilosebaceous unit in the skin.Nat Commun. 2018 Aug 24;9(1):3425. doi: 10.1038/s41467-018-05726-z. Nat Commun. 2018. PMID: 30143626 Free PMC article.
References
-
- Apelqvist, A., Li, H., Sommer, L., Beatus, P., Anderson, D.J., Honjo, T., Hrabe de Angelis, M., Lendahl, U., Edlund, H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. - PubMed
-
- Artavanis-Tsakonas, S., Rand, M.D., Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Bardin, A.J., Le Borgne, R., Schweisguth, F. Asymmetric localization and function of cell-fate determinants: A fly's view. Curr. Opin. Neurobiol. 2004;14:6–14. - PubMed
-
- Barolo, S., Walker, R.G., Polyanovsky, A.D., Freschi, G., Keil, T., Posakony, J.W. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell. 2000;103:957–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
