The ORF7b protein of severe acute respiratory syndrome coronavirus (SARS-CoV) is expressed in virus-infected cells and incorporated into SARS-CoV particles
- PMID: 17079322
- PMCID: PMC1797472
- DOI: 10.1128/JVI.01691-06
The ORF7b protein of severe acute respiratory syndrome coronavirus (SARS-CoV) is expressed in virus-infected cells and incorporated into SARS-CoV particles
Abstract
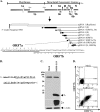
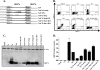
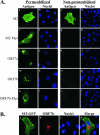
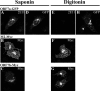
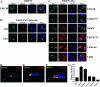
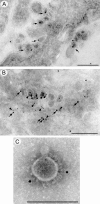
Coronavirus replication is facilitated by a number of highly conserved viral proteins. The viruses also encode accessory genes, which are virus group specific and believed to play roles in virus replication and pathogenesis in vivo. Of the eight putative accessory proteins encoded by the severe acute respiratory distress syndrome associated coronavirus (SARS-CoV), only two-open reading frame 3a (ORF3a) and ORF7a-have been identified in virus-infected cells to date. The ORF7b protein is a putative viral accessory protein encoded on subgenomic (sg) RNA 7. The ORF7b initiation codon overlaps the ORF7a stop codon in a -1 shifted ORF. We demonstrate that the ORF7b protein is expressed in virus-infected cell lysates and from a cDNA encoding the gene 7 coding region, indicating that the sgRNA7 is bicistronic. The translation of ORF7b appears to be mediated by ribosome leaky scanning, and the protein has biochemical properties consistent with that of an integral membrane protein. ORF7b localizes to the Golgi compartment and is incorporated into SARS-CoV particles. We therefore conclude that the ORF7b protein is not only an accessory protein but a structural component of the SARS-CoV virion.
Figures

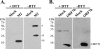

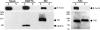
Similar articles
-
The transmembrane domain of the severe acute respiratory syndrome coronavirus ORF7b protein is necessary and sufficient for its retention in the Golgi complex.J Virol. 2008 Oct;82(19):9477-91. doi: 10.1128/JVI.00784-08. Epub 2008 Jul 16. J Virol. 2008. PMID: 18632859 Free PMC article.
-
Severe acute respiratory syndrome coronavirus accessory protein 9b is a virion-associated protein.Virology. 2009 Jun 5;388(2):279-85. doi: 10.1016/j.virol.2009.03.032. Epub 2009 Apr 25. Virology. 2009. PMID: 19394665 Free PMC article.
-
Contribution of SARS-CoV-2 Accessory Proteins to Viral Pathogenicity in K18 Human ACE2 Transgenic Mice.J Virol. 2021 Aug 10;95(17):e0040221. doi: 10.1128/JVI.00402-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133899 Free PMC article.
-
SARS coronavirus accessory proteins.Virus Res. 2008 Apr;133(1):113-21. doi: 10.1016/j.virusres.2007.10.009. Epub 2007 Nov 28. Virus Res. 2008. PMID: 18045721 Free PMC article. Review.
-
The role of SARS-CoV-2 accessory proteins in immune evasion.Biomed Pharmacother. 2022 Dec;156:113889. doi: 10.1016/j.biopha.2022.113889. Epub 2022 Oct 17. Biomed Pharmacother. 2022. PMID: 36265309 Free PMC article. Review.
Cited by
-
Mouse hepatitis virus JHMV I protein is required for maximal virulence.J Virol. 2024 Sep 17;98(9):e0068024. doi: 10.1128/jvi.00680-24. Epub 2024 Aug 19. J Virol. 2024. PMID: 39158347
-
Improved sub-genomic RNA prediction with the ARTIC protocol.Nucleic Acids Res. 2024 Sep 23;52(17):e82. doi: 10.1093/nar/gkae687. Nucleic Acids Res. 2024. PMID: 39149898 Free PMC article.
-
JN.1: ongoing considerations of the shifting landscape of SARS-CoV-2 variants.Future Microbiol. 2024;19(7):559-562. doi: 10.2217/fmb-2024-0010. Epub 2024 Apr 17. Future Microbiol. 2024. PMID: 38629923 No abstract available.
-
The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity.iScience. 2023 Sep 28;26(11):108080. doi: 10.1016/j.isci.2023.108080. eCollection 2023 Nov 17. iScience. 2023. PMID: 37860693 Free PMC article.
-
One for all-human kidney Caki-1 cells are highly susceptible to infection with corona- and other respiratory viruses.J Virol. 2023 Sep 28;97(9):e0055523. doi: 10.1128/jvi.00555-23. Epub 2023 Sep 5. J Virol. 2023. PMID: 37668370 Free PMC article.
References
-
- Astell, C. R., R. A. Holt, S. J. M. Jones, and M. A. Marra. 2005. Genome organization and structural aspects of the SARS-related virus, p. 101-128. In A. Schmidt, M. H. Wolff, and O. Weber (ed.), Coronaviruses with special emphasis on first insights concerning SARS. Birkhauser Verlag, Basel, Switzerland.
-
- Reference deleted.
-
- Reference deleted.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

