Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in Planta
- PMID: 17079311
- PMCID: PMC1797466
- DOI: 10.1128/JVI.01277-06
Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in Planta
Abstract
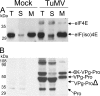
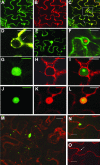
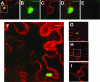
The RNA genome of Turnip mosaic virus is covalently linked at its 5' end to a viral protein known as VPg. This protein binds to the translation eukaryotic initiation factor iso 4E [eIF(iso)4E]. This interaction has been shown to be important for virus infection, although its exact biological function(s) has not been elucidated. In this study, we investigated the subcellular site of the VPg-eIF(iso)4E interaction using bimolecular fluorescence complementation (BiFC). As a first step, eIF(iso)4E, 6K-VPg-Pro, and VPg-Pro were expressed as full-length green fluorescent protein (GFP) fusions in Nicotiana benthamiana, and their subcellular localizations were visualized by confocal microscopy. eIF(iso)4E was predominantly associated with the endoplasmic reticulum (ER), and VPg-Pro was observed in the nucleus and possibly the nucleolus, while 6K-VPg-Pro-GFP induced the formation of cytoplasmic vesicles budding from the ER. In BiFC experiments, reconstituted green fluorescence was observed throughout the nucleus, with a preferential accumulation in subnuclear structures when the GFP split fragments were fused to VPg-Pro and eIF(iso)4E. On the other hand, the interaction of 6K-VPg-Pro with eIF(iso)4E was observed in cytoplasmic vesicles embedded in the ER. These data suggest that the association of VPg with the translation factor might be needed for two different functions, depending of the VPg precursor involved in the interaction. VPg-Pro interaction with eIF(iso)4E may be involved in perturbing normal cellular functions, while 6K-VPg-Pro interaction with the translation factor may be needed for viral RNA translation and/or replication.
Figures
Similar articles
-
Variability in eukaryotic initiation factor iso4E in Brassica rapa influences interactions with the viral protein linked to the genome of Turnip mosaic virus.Sci Rep. 2018 Sep 11;8(1):13588. doi: 10.1038/s41598-018-31739-1. Sci Rep. 2018. PMID: 30206242 Free PMC article.
-
Interaction of VPg-Pro of turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta.J Gen Virol. 2004 Apr;85(Pt 4):1055-1063. doi: 10.1099/vir.0.19706-0. J Gen Virol. 2004. PMID: 15039548
-
Turnip mosaic virus VPg interacts with Arabidopsis thaliana eIF(iso)4E and inhibits in vitro translation.Biochimie. 2008 Oct;90(10):1427-34. doi: 10.1016/j.biochi.2008.03.013. Epub 2008 Jun 6. Biochimie. 2008. PMID: 18582528
-
The genome-linked protein VPg of plant viruses-a protein with many partners.Curr Opin Virol. 2011 Nov;1(5):347-54. doi: 10.1016/j.coviro.2011.09.010. Epub 2011 Oct 14. Curr Opin Virol. 2011. PMID: 22440836 Review.
-
The Molecular Maze of Potyviral and Host Protein Interactions.Annu Rev Virol. 2024 Sep;11(1):147-170. doi: 10.1146/annurev-virology-100422-034124. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38848589 Review.
Cited by
-
Barley Yellow Mosaic Virus VPg Is the Determinant Protein for Breaking eIF4E-Mediated Recessive Resistance in Barley Plants.Front Plant Sci. 2016 Sep 30;7:1449. doi: 10.3389/fpls.2016.01449. eCollection 2016. Front Plant Sci. 2016. PMID: 27746794 Free PMC article.
-
Helper component proteinase of the genus Potyvirus is an interaction partner of translation initiation factors eIF(iso)4E and eIF4E and contains a 4E binding motif.J Virol. 2011 Jul;85(13):6784-94. doi: 10.1128/JVI.00485-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525344 Free PMC article.
-
Intracellular coordination of potyviral RNA functions in infection.Front Plant Sci. 2014 Mar 26;5:110. doi: 10.3389/fpls.2014.00110. eCollection 2014. Front Plant Sci. 2014. PMID: 24723931 Free PMC article. Review.
-
Three-Dimensional Architecture and Biogenesis of Membrane Structures Associated with Plant Virus Replication.Front Plant Sci. 2018 Jan 30;9:57. doi: 10.3389/fpls.2018.00057. eCollection 2018. Front Plant Sci. 2018. PMID: 29441085 Free PMC article. Review.
-
Protein Nucleotidylylation in +ssRNA Viruses.Viruses. 2021 Aug 5;13(8):1549. doi: 10.3390/v13081549. Viruses. 2021. PMID: 34452414 Free PMC article. Review.
References
-
- Anindya, R., and H. S. Savithri. 2004. Potyviral NIa proteinase, a proteinase with novel deoxyribonuclease activity. J. Biol. Chem. 279:32159-32169. - PubMed
-
- Barneche, F., F. Steinmetz, and M. Echeverria. 2000. Fibrillarin genes encode both a conserved nucleolar protein and a novel small nucleolar RNA involved in ribosomal RNA methylation in Arabidopsis thaliana. J. Biol. Chem. 275:27212-27220. - PubMed
-
- Bracha-Drori, K., K. Shichrur, A. Katz, M. Oliva, R. Angelovici, S. Yalovsky, and N. Ohad. 2004. Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J. 40:419-427. - PubMed
-
- Browning, K. S. 1996. The plant translational apparatus. Plant Mol. Biol. 32:107-144. - PubMed
-
- Browning, K. S., C. Webster, J. K. Roberts, and J. M. Ravel. 1992. Identification of an isozyme form of protein synthesis initiation factor 4F in plants. J. Biol. Chem. 267:10096-10100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

