HIV-1 infection and CD4 T cell depletion in the humanized Rag2-/-gamma c-/- (RAG-hu) mouse model
- PMID: 17078891
- PMCID: PMC1635423
- DOI: 10.1186/1742-4690-3-76
HIV-1 infection and CD4 T cell depletion in the humanized Rag2-/-gamma c-/- (RAG-hu) mouse model
Abstract
Background: The currently well-established humanized mouse models, namely the hu-PBL-SCID and SCID-hu systems played an important role in HIV pathogenesis studies. However, despite many notable successes, several limitations still exist. They lack multi-lineage human hematopoiesis and a functional human immune system. These models primarily reflect an acute HIV infection with rapid CD4 T cell loss thus limiting pathogenesis studies to a short-term period. The new humanized Rag2-/-gamma c-/- mouse model (RAG-hu) created by intrahepatic injection of CD34 hematopoietic stem cells sustains long-term multi-lineage human hematopoiesis and is capable of mounting immune responses. Thus, this model shows considerable promise to study long-term in vivo HIV infection and pathogenesis.
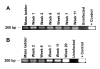
Results: Here we demonstrate that RAG-hu mice produce human cell types permissive to HIV-1 infection and that they can be productively infected by HIV-1 ex vivo. To assess the capacity of these mice to sustain long-term infection in vivo, they were infected by either X4-tropic or R5-tropic HIV-1. Viral infection was assessed by PCR, co-culture, and in situ hybridization. Our results show that both X4 and R5 viruses are capable of infecting RAG-hu mice and that viremia lasts for at least 30 weeks. Moreover, HIV-1 infection leads to CD4 T cell depletion in peripheral blood and thymus, thus mimicking key aspects of HIV-1 pathogenesis. Additionally, a chimeric HIV-1 NL4-3 virus expressing a GFP reporter, although capable of causing viremia, failed to show CD4 T cell depletion possibly due to attenuation.
Conclusion: The humanized RAG-hu mouse model, characterized by its capacity for sustained multi-lineage human hematopoiesis and immune response, can support productive HIV-1 infection. Both T cell and macrophage tropic HIV-1 strains can cause persistent infection of RAG-hu mice resulting in CD4 T cell loss. Prolonged viremia in the context of CD4 T cell depletion seen in this model mirrors the main features of HIV infection in the human. Thus, the RAG-hu mouse model of HIV-1 infection shows great promise for future in vivo pathogenesis studies, evaluation of new drug treatments, vaccines and novel gene therapy strategies.
Figures






Similar articles
-
Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2-/- gammac -/- (RAG-hu) mice.Virology. 2008 Apr 10;373(2):342-51. doi: 10.1016/j.virol.2007.11.020. Epub 2008 Jan 18. Virology. 2008. PMID: 18207484 Free PMC article.
-
Humanized Rag2(-/-)gammac(-/-) (RAG-hu) mice can sustain long-term chronic HIV-1 infection lasting more than a year.Virology. 2010 Feb 5;397(1):100-3. doi: 10.1016/j.virol.2009.10.034. Epub 2009 Nov 18. Virology. 2010. PMID: 19922970 Free PMC article.
-
Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection.J Virol. 1999 Aug;73(8):6453-9. doi: 10.1128/JVI.73.8.6453-6459.1999. J Virol. 1999. PMID: 10400739 Free PMC article.
-
New generation humanized mice for virus research: comparative aspects and future prospects.Virology. 2013 Jan 5;435(1):14-28. doi: 10.1016/j.virol.2012.10.007. Virology. 2013. PMID: 23217612 Free PMC article. Review.
-
Cytopenias in HIV infection: mechanisms and alleviation of hematopoietic inhibition.Curr HIV Res. 2004 Jul;2(3):275-82. doi: 10.2174/1570162043351282. Curr HIV Res. 2004. PMID: 15279591 Review.
Cited by
-
Can humanized mice reflect the complex pathobiology of HIV-associated neurocognitive disorders?J Neuroimmune Pharmacol. 2012 Jun;7(2):352-62. doi: 10.1007/s11481-011-9335-y. Epub 2012 Jan 7. J Neuroimmune Pharmacol. 2012. PMID: 22222956 Free PMC article. Review.
-
Establishing humanized mice using stem cells: maximizing the potential.Clin Exp Immunol. 2008 Jun;152(3):406-14. doi: 10.1111/j.1365-2249.2008.03659.x. Epub 2008 Apr 24. Clin Exp Immunol. 2008. PMID: 18435804 Free PMC article. Review.
-
Humanized mouse models of HIV infection.AIDS Rev. 2011 Jul-Sep;13(3):135-48. AIDS Rev. 2011. PMID: 21799532 Free PMC article. Review.
-
Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2-/-{gamma}c-/- mouse.J Virol. 2009 Aug;83(16):8254-8. doi: 10.1128/JVI.00580-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19494021 Free PMC article.
-
TALEN gene editing takes aim on HIV.Hum Genet. 2016 Sep;135(9):1059-70. doi: 10.1007/s00439-016-1678-2. Epub 2016 May 12. Hum Genet. 2016. PMID: 27170155 Free PMC article. Review.
References
-
- Mosier DE. Human immunodeficiency virus infection of human cells transplanted to severe combined immunodeficient mice. Adv Immun. 1996;63:79–125. - PubMed
-
- Mosier DE, Gulizia RJ, Baird SM, Spector S, Spector D, Kipps TJ, Fox RI, Carson DA, Cooper N, Richman DD, al. Studies of HIV infection and the development of Epstein-Barr virus-related B cell lymphomas following transfer of human lymphocytes to mice with severe combined immunodeficiency. Curr Top Micro Immun. 1989;152:195–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

