DNA polymerase-beta is expressed early in neurons of Alzheimer's disease brain and is loaded into DNA replication forks in neurons challenged with beta-amyloid
- PMID: 17065437
- PMCID: PMC6674652
- DOI: 10.1523/JNEUROSCI.2793-06.2006
DNA polymerase-beta is expressed early in neurons of Alzheimer's disease brain and is loaded into DNA replication forks in neurons challenged with beta-amyloid
Abstract
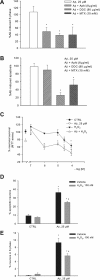
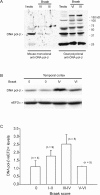
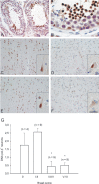
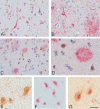
Cultured neurons exposed to synthetic beta-amyloid (Abeta) fragments reenter the cell cycle and initiate a pathway of DNA replication that involves the repair enzyme DNA polymerase-beta (DNA pol-beta) before undergoing apoptotic death. In this study, by performing coimmunoprecipitation experiments on cross-linked nucleoprotein fragments from Abeta-treated neurons, we demonstrate that DNA pol-beta coimmunoprecipitates with cell division cycle 45 (Cdc45) and with DNA primase in short nucleoprotein fragments. This indicates that DNA pol-beta is loaded into neuronal DNA replication forks after Abeta treatment. In response to Abeta the canonical DNA-synthesizing enzyme DNA pol-delta also was loaded into neuronal replication forks, but at later times than DNA pol-beta. Methoxyamine, an inhibitor of the apurinic/apyrimidinic endonuclease that allows for the recruitment of DNA pol-beta during the process of base excision repair (BER), failed to affect coimmunoprecipitation between DNA pol-beta and Cdc45, indicating that DNA pol-beta loading to the replication forks is independent of DNA breaks. However, methoxyamine reduced DNA replication and ensuing apoptosis in neurons exposed to Abeta, suggesting that an efficient BER process allows DNA replication to proceed up to the threshold for death. These data demonstrate that DNA pol-beta is an essential component of the DNA replication machinery in Abeta-treated neurons and additionally support the hypothesis of a close association of cell cycle events with neuronal death in Alzheimer's disease (AD). Accordingly, by investigating the neuronal expression of DNA pol-beta, along with phosphorylated retinoblastoma protein and neurofibrillary changes in AD brain, we show an early involvement of DNA pol-beta in the pathogenesis of AD.
Figures

Similar articles
-
Identification of 5-Methoxyflavone as a Novel DNA Polymerase-Beta Inhibitor and Neuroprotective Agent against Beta-Amyloid Toxicity.J Nat Prod. 2015 Nov 25;78(11):2704-11. doi: 10.1021/acs.jnatprod.5b00621. Epub 2015 Oct 30. J Nat Prod. 2015. PMID: 26517378
-
Molecular Connections between DNA Replication and Cell Death in β-Amyloid-Treated Neurons.Curr Neuropharmacol. 2023;21(9):2006-2018. doi: 10.2174/1570159X21666230404121903. Curr Neuropharmacol. 2023. PMID: 37021419 Free PMC article.
-
Transient OGG1, APE1, PARP1 and Polβ expression in an Alzheimer's disease mouse model.Mech Ageing Dev. 2013 Oct;134(10):467-77. doi: 10.1016/j.mad.2013.09.002. Epub 2013 Oct 11. Mech Ageing Dev. 2013. PMID: 24121118
-
The nature of the cell cycle in neurons: focus on a "non-canonical" pathway of DNA replication causally related to death.Biochim Biophys Acta. 2007 Apr;1772(4):409-12. doi: 10.1016/j.bbadis.2006.10.016. Epub 2006 Nov 1. Biochim Biophys Acta. 2007. PMID: 17196375 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
The active components of Erzhi wan and their anti-Alzheimer's disease mechanisms determined by an integrative approach of network pharmacology, bioinformatics, molecular docking, and molecular dynamics simulation.Heliyon. 2024 Jun 27;10(13):e33761. doi: 10.1016/j.heliyon.2024.e33761. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027618 Free PMC article.
-
TGF-β1 pathway as a new target for neuroprotection in Alzheimer's disease.CNS Neurosci Ther. 2011 Aug;17(4):237-49. doi: 10.1111/j.1755-5949.2009.00115.x. Epub 2009 Nov 19. CNS Neurosci Ther. 2011. PMID: 19925479 Free PMC article. Review.
-
Into the Fourth Dimension: Dysregulation of Genome Architecture in Aging and Alzheimer's Disease.Front Mol Neurosci. 2018 Feb 28;11:60. doi: 10.3389/fnmol.2018.00060. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29541020 Free PMC article. Review.
-
Mutagenic Replication of the Major Oxidative Adenine Lesion 7,8-Dihydro-8-oxoadenine by Human DNA Polymerases.J Am Chem Soc. 2019 Mar 20;141(11):4584-4596. doi: 10.1021/jacs.8b08551. Epub 2019 Mar 7. J Am Chem Soc. 2019. PMID: 30817143 Free PMC article.
-
Senescence as an Amyloid Cascade: The Amyloid Senescence Hypothesis.Front Cell Neurosci. 2020 May 19;14:129. doi: 10.3389/fncel.2020.00129. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32508595 Free PMC article. Review.
References
-
- Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Progr Neurobiol. 2004;72:1–25. - PubMed
-
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. - PubMed
-
- Copani A, Condorelli F, Caruso A, Vancheri C, Sala A, Giuffrida Stella AM, Canonico PL, Nicoletti F, Sortino MA. Mitotic signaling by beta amyloid causes neuronal death. FASEB J. 1999;13:2225–2234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
