Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid
- PMID: 17056734
- PMCID: PMC1751408
- DOI: 10.1529/biophysj.106.091876
Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid
Abstract
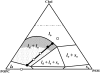
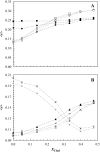
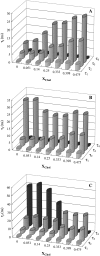
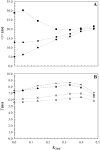
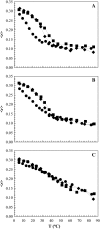
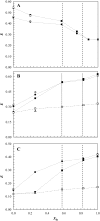
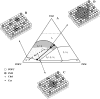
The effect of physiologically relevant ceramide concentrations (< or = 4 mol %) in raft model membranes with a lipid composition resembling that of cell membranes, i.e., composed of different molar ratios of an unsaturated glycerophospholipid, sphingomyelin, and cholesterol (Chol) along a liquid-disordered-liquid-ordered tie line was explored. The application of a fluorescence multiprobe and multiparameter approach, together with multiple fluorescence resonance energy transfer (FRET) pairs, in the well-characterized palmitoyl-oleoyl-phosphocholine (POPC)/palmitoyl-sphingomyelin (PSM)/Chol ternary mixture, revealed that low palmitoyl-ceramide (PCer) concentrations strongly changed both the biophysical properties and lipid lateral organization of the ternary mixtures in the low-to-intermediate Chol/PSM-, small raft size range (<25 mol % Chol). For these mixtures, PCer recruited up to three PSM molecules for the formation of very small ( approximately 4 nm) and highly ordered gel domains, which became surrounded by rafts (liquid-ordered phase) when Chol/PSM content increased. However, the size of these rafts did not change, showing that PCer did not induce the formation of large platforms or the coalescence of small rafts. In the high Chol/PSM-, large raft domains range (>33 mol % Chol), Chol completely abolished the effect of PCer by competing for PSM association. Lipid rafts govern the biophysical properties and lateral organization in these last mixtures.
Figures
Similar articles
-
Formation of ceramide/sphingomyelin gel domains in the presence of an unsaturated phospholipid: a quantitative multiprobe approach.Biophys J. 2007 Sep 1;93(5):1639-50. doi: 10.1529/biophysj.107.107714. Epub 2007 May 11. Biophys J. 2007. PMID: 17496019 Free PMC article.
-
A combined fluorescence spectroscopy, confocal and 2-photon microscopy approach to re-evaluate the properties of sphingolipid domains.Biochim Biophys Acta. 2013 Sep;1828(9):2099-110. doi: 10.1016/j.bbamem.2013.05.011. Epub 2013 May 20. Biochim Biophys Acta. 2013. PMID: 23702462
-
Characterization of the ternary mixture of sphingomyelin, POPC, and cholesterol: support for an inhomogeneous lipid distribution at high temperatures.Biophys J. 2008 Apr 1;94(7):2680-90. doi: 10.1529/biophysj.107.112904. Epub 2008 Jan 4. Biophys J. 2008. PMID: 18178660 Free PMC article.
-
Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies.Biochim Biophys Acta. 2005 Dec 30;1746(3):193-202. doi: 10.1016/j.bbamcr.2005.09.003. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16271405 Review.
-
Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy.Biochim Biophys Acta. 2003 Mar 10;1610(2):231-43. doi: 10.1016/s0005-2736(03)00021-x. Biochim Biophys Acta. 2003. PMID: 12648777 Review.
Cited by
-
Complexity of lipid domains and rafts in giant unilamellar vesicles revealed by combining imaging and microscopic and macroscopic time-resolved fluorescence.Biophys J. 2007 Jul 15;93(2):539-53. doi: 10.1529/biophysj.106.098822. Epub 2007 Apr 20. Biophys J. 2007. PMID: 17449668 Free PMC article.
-
Influence of ceramide on lipid domain stability studied with small-angle neutron scattering: The role of acyl chain length and unsaturation.Chem Phys Lipids. 2022 Jul;245:105205. doi: 10.1016/j.chemphyslip.2022.105205. Epub 2022 Apr 26. Chem Phys Lipids. 2022. PMID: 35483419 Free PMC article.
-
Sequence of physical changes to the cell membrane during glucocorticoid-induced apoptosis in S49 lymphoma cells.Biophys J. 2009 Apr 8;96(7):2709-18. doi: 10.1016/j.bpj.2008.12.3925. Biophys J. 2009. PMID: 19348753 Free PMC article.
-
Secondary Ion Mass Spectrometry Imaging Reveals Changes in the Lipid Structure of the Plasma Membranes of Hippocampal Neurons following Drugs Affecting Neuronal Activity.ACS Chem Neurosci. 2021 May 5;12(9):1542-1551. doi: 10.1021/acschemneuro.1c00031. Epub 2021 Apr 26. ACS Chem Neurosci. 2021. PMID: 33896172 Free PMC article.
-
State of the Art in Stratum Corneum Research. Part II: Hypothetical Stratum Corneum Lipid Matrix Models.Skin Pharmacol Physiol. 2020;33(4):213-230. doi: 10.1159/000509019. Epub 2020 Jul 17. Skin Pharmacol Physiol. 2020. PMID: 32683377 Free PMC article. Review.
References
-
- Kusumi, A., C. Nakada, K. Ritchie, K. Murase, K. Suzuki, H. Murakoshi, R. S. Kasai, J. Kondo, and T. Fujiwara. 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomolec. Struct. 34:351–378. - PubMed
-
- Karnovsky, M. J., A. M. Kleinfeld, R. L. Hoover, E. A. Dawidowicz, D. E. McIntyre, E. A. Salzman, and R. D. Klausner. 1982. Lipid domains in membranes. Ann. N. Y. Acad. Sci. 401:61–75. - PubMed
-
- Estep, T. N., D. B. Mountcastle, Y. Barenholz, R. L. Biltonen, and T. E. Thompson. 1979. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 18:2112–2117. - PubMed
-
- Ipsen, J. H., G. Karlstrom, O. G. Mouritsen, H. Wennerstrom, and M. J. Zuckermann. 1987. Phase-equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 905:162–172. - PubMed
-
- Sankaram, M. B., and T. E. Thompson. 1990. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 29:10670–10675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

