Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I
- PMID: 17050612
- PMCID: PMC1797271
- DOI: 10.1128/JVI.01134-06
Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I
Abstract
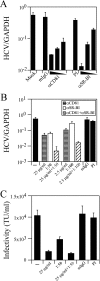
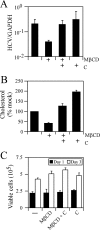
In the past several years, a number of cellular proteins have been identified as candidate entry receptors for hepatitis C virus (HCV) by using surrogate models of HCV infection. Among these, the tetraspanin CD81 and scavenger receptor B type I (SR-BI), both of which localize to specialized plasma membrane domains enriched in cholesterol, have been suggested to be key players in HCV entry. In the current study, we used a recently developed in vitro HCV infection system to demonstrate that both CD81 and SR-BI are required for authentic HCV infection in vitro, that they function cooperatively to initiate HCV infection, and that CD81-mediated HCV entry is, in part, dependent on membrane cholesterol.
Figures

Similar articles
-
The level of CD81 cell surface expression is a key determinant for productive entry of hepatitis C virus into host cells.J Virol. 2007 Jan;81(2):588-98. doi: 10.1128/JVI.01534-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079281 Free PMC article.
-
Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra- and extra-cellular domains.PLoS Pathog. 2009 Feb;5(2):e1000310. doi: 10.1371/journal.ppat.1000310. Epub 2009 Feb 20. PLoS Pathog. 2009. PMID: 19229312 Free PMC article.
-
Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor.J Biol Chem. 2003 Oct 24;278(43):41624-30. doi: 10.1074/jbc.M305289200. Epub 2003 Aug 11. J Biol Chem. 2003. PMID: 12913001
-
Hepatitis C virus entry into host cells.Cell Mol Life Sci. 2008 Jan;65(1):100-12. doi: 10.1007/s00018-007-7291-8. Cell Mol Life Sci. 2008. PMID: 17914604 Free PMC article. Review.
-
Hepatitis C virus entry: potential receptors and their biological functions.J Gen Virol. 2006 May;87(Pt 5):1075-1084. doi: 10.1099/vir.0.81646-0. J Gen Virol. 2006. PMID: 16603507 Review.
Cited by
-
Augmentation of 3β-hydroxysteroid-Δ24 Reductase (DHCR24) Expression Induced by Bovine Viral Diarrhea Virus Infection Facilitates Viral Replication via Promoting Cholesterol Synthesis.J Virol. 2022 Dec 21;96(24):e0149222. doi: 10.1128/jvi.01492-22. Epub 2022 Dec 5. J Virol. 2022. PMID: 36468862 Free PMC article.
-
Production, purification and characterization of recombinant, full-length human claudin-1.PLoS One. 2013 May 21;8(5):e64517. doi: 10.1371/journal.pone.0064517. Print 2013. PLoS One. 2013. PMID: 23704991 Free PMC article.
-
Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms.J Virol. 2010 Jan;84(1):666-70. doi: 10.1128/JVI.01156-09. J Virol. 2010. PMID: 19846523 Free PMC article.
-
Adaptive immunity to the hepatitis C virus.Adv Virus Res. 2010;78:43-86. doi: 10.1016/B978-0-12-385032-4.00002-1. Adv Virus Res. 2010. PMID: 21040831 Free PMC article. Review.
-
Hypervariable Region 1 in Envelope Protein 2 of Hepatitis C Virus: A Linchpin in Neutralizing Antibody Evasion and Viral Entry.Front Immunol. 2018 Sep 27;9:2146. doi: 10.3389/fimmu.2018.02146. eCollection 2018. Front Immunol. 2018. PMID: 30319614 Free PMC article. Review.
References
-
- Allander, T., X. Forns, S. U. Emerson, R. H. Purcell, and J. Bukh. 2000. Hepatitis C virus envelope protein E2 binds to CD81 of tamarins. Virology 277:358-367. - PubMed
-
- Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, R. E. Sampliner, et al. 1992. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N. Engl. J. Med. 327:1899-1905. - PubMed
-
- Babitt, J., B. Trigatti, A. Rigotti, E. J. Smart, R. G. Anderson, S. Xu, and M. Krieger. 1997. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J. Biol. Chem. 272:13242-13249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

