Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases
- PMID: 17041104
- PMCID: PMC2228334
- DOI: 10.1158/1535-7163.MCT-06-0310
Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases
Abstract
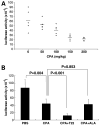
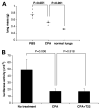
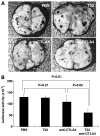
Expression of the chemokine receptor CXCR4 by tumor cells promotes metastasis, possibly by activating prosurvival signals that render cancer cells resistant to immune attack. Inhibition of CXCR4 with a peptide antagonist, T22, blocks metastatic implantation of CXCR4-transduced B16 (CXCR4-luc-B16) melanoma cells in lung, but not the outgrowth of established metastases, raising the question of how T22 can best be used in a clinical setting. Herein, whereas the treatment of CXCR4-luc-B16 cells in vitro with the CXCR4 ligand CXCL12 did not reduce killing induced by cisplatin or cyclophosphamide, CXCL12 markedly reduced Fas-dependent killing by gp100-specific (pmel-1) CD8(+) T cells. T22 pretreatment restored sensitivity of CXCR4-luc-B16 cells to pmel-1 killing, even in the presence of CXCL12. Two immune-augmenting regimens were used in combination with T22 to treat experimental lung metastases. First, low-dose cyclophosphamide treatment (100 mg/kg) on day 5 in combination with T22 (days 4-7) yielded a approximately 70% reduction of B16 metastatic tumor burden in the lungs compared with cyclophosphamide treatment alone (P < 0.001). Furthermore, whereas anti-CTL antigen 4 (CTLA4) monoclonal antibody (mAb; or T22 treatment) alone had little effect on established B16 metastases, pretreatment with T22 (in combination with anti-CTLA4 mAb) resulted in a 50% reduction in lung tumor burden (P = 0.02). Thus, in vitro, CXCR4 antagonism with T22 renders B16 cells susceptible to killing by antigen-specific T cells. In vivo, T22 synergizes with cyclophosphamide or anti-CTLA4 mAb in the treatment of established lung metastases, suggesting a novel strategy for augmenting the efficacy of immunotherapy.
Figures
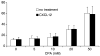
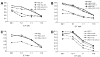
Similar articles
-
Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells.Cancer Res. 2002 Dec 15;62(24):7328-34. Cancer Res. 2002. PMID: 12499276
-
Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control.J Immunol. 2006 Mar 1;176(5):2902-14. doi: 10.4049/jimmunol.176.5.2902. J Immunol. 2006. PMID: 16493048
-
CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin.Cancer Res. 2003 Oct 15;63(20):6751-7. Cancer Res. 2003. PMID: 14583470
-
Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation.J Exp Med. 1999 Aug 2;190(3):355-66. doi: 10.1084/jem.190.3.355. J Exp Med. 1999. PMID: 10430624 Free PMC article.
-
The involvement of a chemokine receptor antagonist CTCE-9908 and kynurenine metabolites in cancer development.Cell Biochem Funct. 2022 Aug;40(6):608-622. doi: 10.1002/cbf.3731. Epub 2022 Jul 5. Cell Biochem Funct. 2022. PMID: 35789495 Review.
Cited by
-
Immunotherapy for advanced melanoma.J Invest Dermatol. 2008 Nov;128(11):2596-2605. doi: 10.1038/jid.2008.101. J Invest Dermatol. 2008. PMID: 18927541 Free PMC article. Review.
-
Vascular dysfunction and increased metastasis of B16F10 melanomas in Shb deficient mice as compared with their wild type counterparts.BMC Cancer. 2015 Apr 8;15:234. doi: 10.1186/s12885-015-1269-y. BMC Cancer. 2015. PMID: 25885274 Free PMC article.
-
Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases.Clin Exp Metastasis. 2008;25(3):201-11. doi: 10.1007/s10585-007-9133-3. Epub 2007 Dec 11. Clin Exp Metastasis. 2008. PMID: 18071913 Free PMC article.
-
Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways.Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17655-60. doi: 10.1073/pnas.1101133108. Epub 2011 Oct 11. Proc Natl Acad Sci U S A. 2011. PMID: 21990345 Free PMC article.
-
CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer.Cancer Res. 2011 Aug 15;71(16):5522-5534. doi: 10.1158/0008-5472.CAN-10-3143. Epub 2011 Jul 8. Cancer Res. 2011. PMID: 21742774 Free PMC article.
References
-
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and meta-static tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

