CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure
- PMID: 17032064
- PMCID: PMC1592316
- DOI: 10.1371/journal.pbio.0040327
CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure
Abstract
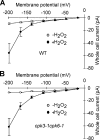
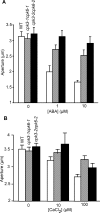
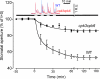
Abscisic acid (ABA) signal transduction has been proposed to utilize cytosolic Ca(2+) in guard cell ion channel regulation. However, genetic mutants in Ca(2+) sensors that impair guard cell or plant ion channel signaling responses have not been identified, and whether Ca(2+)-independent ABA signaling mechanisms suffice for a full response remains unclear. Calcium-dependent protein kinases (CDPKs) have been proposed to contribute to central signal transduction responses in plants. However, no Arabidopsis CDPK gene disruption mutant phenotype has been reported to date, likely due to overlapping redundancies in CDPKs. Two Arabidopsis guard cell-expressed CDPK genes, CPK3 and CPK6, showed gene disruption phenotypes. ABA and Ca(2+) activation of slow-type anion channels and, interestingly, ABA activation of plasma membrane Ca(2+)-permeable channels were impaired in independent alleles of single and double cpk3cpk6 mutant guard cells. Furthermore, ABA- and Ca(2+)-induced stomatal closing were partially impaired in these cpk3cpk6 mutant alleles. However, rapid-type anion channel current activity was not affected, consistent with the partial stomatal closing response in double mutants via a proposed branched signaling network. Imposed Ca(2+) oscillation experiments revealed that Ca(2+)-reactive stomatal closure was reduced in CDPK double mutant plants. However, long-lasting Ca(2+)-programmed stomatal closure was not impaired, providing genetic evidence for a functional separation of these two modes of Ca(2+)-induced stomatal closing. Our findings show important functions of the CPK6 and CPK3 CDPKs in guard cell ion channel regulation and provide genetic evidence for calcium sensors that transduce stomatal ABA signaling.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures


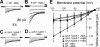


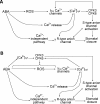
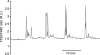
Comment in
-
Protein kinases and plant pores.PLoS Biol. 2006 Oct;4(10):e358. doi: 10.1371/journal.pbio.0040358. Epub 2006 Oct 10. PLoS Biol. 2006. PMID: 20076480 Free PMC article. No abstract available.
Similar articles
-
Calcium-dependent protein kinase CPK9 negatively functions in stomatal abscisic acid signaling by regulating ion channel activity in Arabidopsis.Plant Mol Biol. 2019 Jan;99(1-2):113-122. doi: 10.1007/s11103-018-0805-y. Epub 2018 Dec 8. Plant Mol Biol. 2019. PMID: 30536042
-
The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells.Plant Physiol. 2011 Jan;155(1):553-61. doi: 10.1104/pp.110.162750. Epub 2010 Oct 26. Plant Physiol. 2011. PMID: 20978156 Free PMC article.
-
The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production.Plant Physiol. 2007 Mar;143(3):1398-407. doi: 10.1104/pp.106.091298. Epub 2007 Jan 12. Plant Physiol. 2007. PMID: 17220365 Free PMC article.
-
Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses.Ann Bot. 2012 Jan;109(1):5-17. doi: 10.1093/aob/mcr252. Epub 2011 Oct 12. Ann Bot. 2012. PMID: 21994053 Free PMC article. Review.
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
Cited by
-
Transcriptome profiling of Arabidopsis slac1-3 mutant reveals compensatory alterations in gene expression underlying defective stomatal closure.Front Plant Sci. 2022 Sep 20;13:987606. doi: 10.3389/fpls.2022.987606. eCollection 2022. Front Plant Sci. 2022. PMID: 36204078 Free PMC article.
-
Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings.Plant Mol Biol. 2013 Mar;81(4-5):347-61. doi: 10.1007/s11103-012-0006-z. Epub 2013 Jan 18. Plant Mol Biol. 2013. PMID: 23329372
-
The Cyclophilin ROC3 Regulates ABA-Induced Stomatal Closure and the Drought Stress Response of Arabidopsis thaliana.Front Plant Sci. 2021 May 25;12:668792. doi: 10.3389/fpls.2021.668792. eCollection 2021. Front Plant Sci. 2021. PMID: 34113366 Free PMC article.
-
Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates.Front Plant Sci. 2011 Aug 30;2:36. doi: 10.3389/fpls.2011.00036. eCollection 2011. Front Plant Sci. 2011. PMID: 22645532 Free PMC article.
-
Regulation of stomatal aperture in response to drought stress mediating with polyamines, nitric oxide synthase and hydrogen peroxide in Rosa canina L.Plant Signal Behav. 2020 Sep 1;15(9):1790844. doi: 10.1080/15592324.2020.1790844. Epub 2020 Jul 11. Plant Signal Behav. 2020. PMID: 32657206 Free PMC article.
References
-
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. - PubMed
-
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. - PubMed
-
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

