The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK
- PMID: 17030606
- PMCID: PMC1800641
- DOI: 10.1128/MCB.01456-06
The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK
Abstract
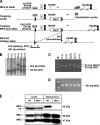
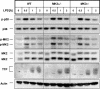
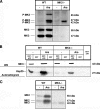
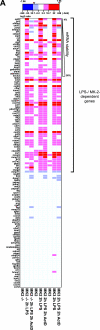
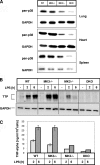
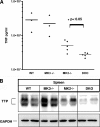
MK2 and MK3 represent protein kinases downstream of p38 mitogen-activated protein kinase (MAPK). Deletion of the MK2 gene in mice resulted in an impaired inflammatory response although MK3, which displays extensive structural similarities and identical functional properties in vitro, is still present. Here, we analyze tumor necrosis factor (TNF) production and expression of p38 MAPK and tristetraprolin (TTP) in MK3-deficient mice and demonstrate that there are no significant differences with wild-type animals. We show that in vivo MK2 and MK3 are expressed and activated in parallel. However, the level of activity of MK2 is always significantly higher than that of MK3. Accordingly, we hypothesized that MK3 could have significant effects only in an MK2-free background and generated MK2/MK3 double-knockout mice. Unexpectedly, these mice are viable and show no obvious defects due to loss of compensation between MK2 and MK3. However, there is a further reduction of TNF production and expression of p38 and TTP in double-knockout mice compared to MK2-deficient mice. This finding, together with the observation that ectopically expressed MK3 can rescue MK2 deficiency similarly to MK2, indicates that both kinases share the same physiological function in vivo but are expressed to different levels.
Figures



Similar articles
-
Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element.Mol Cell Biol. 2006 Mar;26(6):2399-407. doi: 10.1128/MCB.26.6.2399-2407.2006. Mol Cell Biol. 2006. PMID: 16508014 Free PMC article.
-
Cytokine regulation by MAPK activated kinase 2 in keratinocytes exposed to sulfur mustard.Toxicol In Vitro. 2013 Oct;27(7):2067-75. doi: 10.1016/j.tiv.2013.07.002. Epub 2013 Jul 10. Toxicol In Vitro. 2013. PMID: 23851002
-
MAPKAP kinases MK2 and MK3 in inflammation: complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin.Biochem Pharmacol. 2010 Dec 15;80(12):1915-20. doi: 10.1016/j.bcp.2010.06.021. Epub 2010 Jun 23. Biochem Pharmacol. 2010. PMID: 20599781 Review.
-
Crucial roles of the protein kinases MK2 and MK3 in a mouse model of glomerulonephritis.PLoS One. 2013;8(1):e54239. doi: 10.1371/journal.pone.0054239. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23372691 Free PMC article.
-
MK2 and MK3--a pair of isoenzymes?Front Biosci. 2008 May 1;13:5511-21. doi: 10.2741/3095. Front Biosci. 2008. PMID: 18508601 Review.
Cited by
-
Proximal inhibition of p38 MAPK stress signaling prevents distal axonopathy.Neurobiol Dis. 2013 Nov;59:26-37. doi: 10.1016/j.nbd.2013.07.001. Epub 2013 Jul 13. Neurobiol Dis. 2013. PMID: 23859799 Free PMC article.
-
MK3 modulation affects BMI1-dependent and independent cell cycle check-points.PLoS One. 2015 Apr 8;10(4):e0118840. doi: 10.1371/journal.pone.0118840. eCollection 2015. PLoS One. 2015. PMID: 25853770 Free PMC article.
-
Spatial subsetting enables integrative modeling of oral squamous cell carcinoma multiplex imaging data.iScience. 2023 Nov 20;26(12):108486. doi: 10.1016/j.isci.2023.108486. eCollection 2023 Dec 15. iScience. 2023. PMID: 38125025 Free PMC article.
-
P38 mitogen-activated protein kinase activity is required during mitosis for timely satisfaction of the mitotic checkpoint but not for the fidelity of chromosome segregation.Mol Biol Cell. 2010 Jul 1;21(13):2150-60. doi: 10.1091/mbc.e10-02-0125. Epub 2010 May 12. Mol Biol Cell. 2010. PMID: 20462950 Free PMC article.
-
Phosphorylation of the small heat shock protein HspB1 regulates cytoskeletal recruitment and cell motility.Mol Biol Cell. 2022 Sep 15;33(11):ar100. doi: 10.1091/mbc.E22-02-0057. Epub 2022 Jun 29. Mol Biol Cell. 2022. PMID: 35767320 Free PMC article.
References
-
- Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. - PubMed
-
- Ben-Levy, R., S. Hooper, R. Wilson, H. F. Paterson, and C. J. Marshall. 1998. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 8:1049-1057. - PubMed
-
- Brook, M., C. R. Tchen, T. Santalucia, J. McIlrath, J. S. Arthur, J. Saklatvala, and A. R. Clark. 2006. Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways. Mol. Cell. Biol. 26:2408-2418. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
