Transition of galactosyltransferase 1 from trans-Golgi cisterna to the trans-Golgi network is signal mediated
- PMID: 17021253
- PMCID: PMC1679680
- DOI: 10.1091/mbc.e06-08-0665
Transition of galactosyltransferase 1 from trans-Golgi cisterna to the trans-Golgi network is signal mediated
Abstract
The Golgi apparatus (GA) is the organelle where complex glycan formation takes place. In addition, it is a major sorting site for proteins destined for various subcellular compartments or for secretion. Here we investigate beta1,4-galactosyltransferase 1 (galT) and alpha2,6-sialyltransferase 1 (siaT), two trans-Golgi glycosyltransferases, with respect to their different pathways in monensin-treated cells. Upon addition of monensin galT dissociates from siaT and the GA and accumulates in swollen vesicles derived from the trans-Golgi network (TGN), as shown by colocalization with TGN46, a specific TGN marker. We analyzed various chimeric constructs of galT and siaT by confocal fluorescence microscopy and time-lapse videomicroscopy as well as Optiprep density gradient fractionation. We show that the first 13 amino acids of the cytoplasmic tail of galT are necessary for its localization to swollen vesicles induced by monensin. We also show that the monensin sensitivity resulting from the cytoplasmic tail can be conferred to siaT, which leads to the rapid accumulation of the galT-siaT chimera in swollen vesicles upon monensin treatment. On the basis of these data, we suggest that cycling between the trans-Golgi cisterna and the trans-Golgi network of galT is signal mediated.
Figures



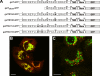
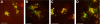
Similar articles
-
Dissection of a novel molecular determinant mediating Golgi to trans-Golgi network transition.Cell Mol Life Sci. 2008 Nov;65(22):3677-87. doi: 10.1007/s00018-008-8446-y. Cell Mol Life Sci. 2008. PMID: 18953688 Free PMC article.
-
Modulation of GalT1 and SialT1 sub-Golgi localization by SialT2 expression reveals an organellar level of glycolipid synthesis control.J Biol Chem. 2006 Oct 27;281(43):32852-60. doi: 10.1074/jbc.M605805200. Epub 2006 Sep 1. J Biol Chem. 2006. PMID: 16950784
-
Actin and microtubule regulation of trans-Golgi network architecture, and copper-dependent protein transport to the cell surface.Mol Membr Biol. 2004 Jan-Feb;21(1):59-66. doi: 10.1080/096870310001607350. Mol Membr Biol. 2004. PMID: 14668139
-
Mammalian GRIP domain proteins differ in their membrane binding properties and are recruited to distinct domains of the TGN.J Cell Sci. 2004 Nov 15;117(Pt 24):5865-74. doi: 10.1242/jcs.01497. Epub 2004 Nov 2. J Cell Sci. 2004. PMID: 15522892
-
The mysterious life of the plant trans-Golgi network: advances and tools to understand it better.J Microsc. 2020 Jun;278(3):154-163. doi: 10.1111/jmi.12881. Epub 2020 Mar 20. J Microsc. 2020. PMID: 32115699 Review.
Cited by
-
Golgi N-glycosyltransferases form both homo- and heterodimeric enzyme complexes in live cells.J Biol Chem. 2010 Jun 4;285(23):17771-7. doi: 10.1074/jbc.M110.103184. Epub 2010 Apr 8. J Biol Chem. 2010. PMID: 20378551 Free PMC article.
-
CellCognition: time-resolved phenotype annotation in high-throughput live cell imaging.Nat Methods. 2010 Sep;7(9):747-54. doi: 10.1038/nmeth.1486. Epub 2010 Aug 8. Nat Methods. 2010. PMID: 20693996
-
Regulation of Golgi structure and secretion by receptor-induced G protein βγ complex translocation.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11417-22. doi: 10.1073/pnas.1003042107. Epub 2010 Jun 7. Proc Natl Acad Sci U S A. 2010. PMID: 20534534 Free PMC article.
-
p125A exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport.J Cell Biol. 2010 Aug 9;190(3):331-45. doi: 10.1083/jcb.201003005. Epub 2010 Aug 2. J Cell Biol. 2010. PMID: 20679433 Free PMC article.
-
Optimizing production of Fc-amidated peptides by Chinese hamster ovary cells.BMC Biotechnol. 2015 Oct 16;15:95. doi: 10.1186/s12896-015-0210-4. BMC Biotechnol. 2015. PMID: 26475607 Free PMC article.
References
-
- Aridor M., Traub L. M. Cargo selection in vesicular transport: the making and breaking of a coat. Traffic. 2002;3:537–546. - PubMed
-
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch. Biochem. Biophys. 1985;240:413–425. - PubMed
-
- Banting G., Ponnambalam S. TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim. Biophys. Acta. 1997;1355:209–217. - PubMed
-
- Barlowe C. COPII: a membrane coat that forms endoplasmic reticulum-derived vesicles. FEBS Lett. 1995;369:93–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

