Interaction of mammalian Hsp22 with lipid membranes
- PMID: 17020537
- PMCID: PMC1820815
- DOI: 10.1042/BJ20061046
Interaction of mammalian Hsp22 with lipid membranes
Abstract
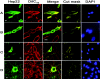
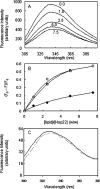
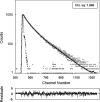
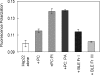
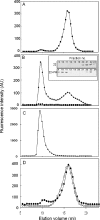
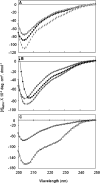
Hsp22/HspB8 is a member of the small heat-shock protein family, whose function is not yet completely understood. Our immunolocalization studies in a human neuroblastoma cell line, SK-N-SH, using confocal microscopy show that a significant fraction of Hsp22 is localized to the plasma membrane. We therefore investigated its interactions with lipid vesicles in vitro. Intrinsic tryptophan fluorescence is quenched in the presence of lipid vesicles derived from either bovine brain lipid extract or purified lipids. Time-resolved fluorescence studies show a decrease in the lifetimes of the tryptophan residues. Both of these results indicate burial of some tryptophan residues of Hsp22 upon interaction with lipid vesicles. Membrane interactions also lead to increase in fluorescence polarization of Hsp22. Gel-filtration chromatography shows that Hsp22 binds stably with lipid vesicles; the extent of binding depends on the nature of the lipid. Hsp22 binds more strongly to vesicles made of lipids containing a phosphatidic acid, phosphatidylinositol or phosphatidylserine headgroup (known to be present in the inner leaflet of plasma membrane) compared with lipid vesicles made of a phosphatidylcholine head-group alone. Far-UV CD spectra reveal conformational changes upon binding to the lipid vesicles or in membrane-mimetic solvent, trifluoroethanol. Thus our fluorescence, CD and gel-filtration studies show that Hsp22 interacts with membrane and this interaction leads to stable binding and conformational changes. The present study therefore clearly demonstrates that Hsp22 exhibits potential membrane interaction that may play an important role in its cellular functions.
Figures
Similar articles
-
Mammalian Hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity.Biochem J. 2004 Jul 15;381(Pt 2):379-87. doi: 10.1042/BJ20031958. Biochem J. 2004. PMID: 15030316 Free PMC article.
-
Interaction of human HSP22 (HSPB8) with other small heat shock proteins.J Biol Chem. 2004 Jan 23;279(4):2394-402. doi: 10.1074/jbc.M311324200. Epub 2003 Oct 31. J Biol Chem. 2004. PMID: 14594798
-
Interaction of heat shock protein 90 B1 (Hsp90B1) with liposome reveals its potential role in protection the integrity of lipid membranes.Int J Biol Macromol. 2018 Jan;106:1250-1257. doi: 10.1016/j.ijbiomac.2017.08.121. Epub 2017 Aug 26. Int J Biol Macromol. 2018. PMID: 28851640
-
Structure, properties, and functions of the human small heat-shock protein HSP22 (HspB8, H11, E2IG1): a critical review.J Neurosci Res. 2008 Feb 1;86(2):264-9. doi: 10.1002/jnr.21441. J Neurosci Res. 2008. PMID: 17722063 Review.
-
Heat shock proteins and the biogenesis of cellular membranes.Cell Stress Chaperones. 2021 Jan;26(1):15-18. doi: 10.1007/s12192-020-01173-2. Epub 2020 Oct 20. Cell Stress Chaperones. 2021. PMID: 33083932 Free PMC article. Review.
Cited by
-
Hold me tight: Role of the heat shock protein family of chaperones in cardiac disease.Circulation. 2010 Oct 26;122(17):1740-51. doi: 10.1161/CIRCULATIONAHA.110.942250. Circulation. 2010. PMID: 20975010 Free PMC article. Review. No abstract available.
-
HspB1, HspB5 and HspB4 in Human Cancers: Potent Oncogenic Role of Some of Their Client Proteins.Cancers (Basel). 2014 Feb 7;6(1):333-65. doi: 10.3390/cancers6010333. Cancers (Basel). 2014. PMID: 24514166 Free PMC article.
-
Membrane-Associated Heat Shock Proteins in Oncology: From Basic Research to New Theranostic Targets.Cells. 2020 May 20;9(5):1263. doi: 10.3390/cells9051263. Cells. 2020. PMID: 32443761 Free PMC article. Review.
-
In vitro study on the interaction between the 32 kDa enamelin and amelogenin.J Struct Biol. 2009 Apr;166(1):88-94. doi: 10.1016/j.jsb.2009.01.003. J Struct Biol. 2009. PMID: 19263522 Free PMC article.
-
Charcot-Marie-Tooth disease and intracellular traffic.Prog Neurobiol. 2012 Dec;99(3):191-225. doi: 10.1016/j.pneurobio.2012.03.003. Epub 2012 Mar 22. Prog Neurobiol. 2012. PMID: 22465036 Free PMC article. Review.
References
-
- Parcellier A., Gurbuxani S., Schmitt E., Solary E., Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem. Biophys. Res. Commun. 2003;304:505–512. - PubMed
-
- Arrigo P. In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J. Cell. Biochem. 2005;94:241–246. - PubMed
-
- Garrido C., Gurbuxani S., Ravagnan L., Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem. Biophys. Res Commun. 2001;286:433–442. - PubMed
-
- Smith C. C., Yu Y. X., Kulka M., Aurelian L. A novel human gene similar to the protein kinase (PK) coding domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) codes for a serine-threonine PK and is expressed in melanoma cells. J. Biol. Chem. 2000;275:25690–25699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

