Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells
- PMID: 17016559
- PMCID: PMC1578632
- DOI: 10.1172/JCI28828
Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells
Abstract
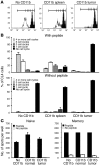
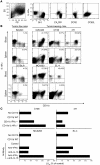
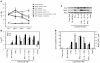
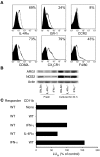
Active suppression of tumor-specific T lymphocytes can limit the efficacy of immune surveillance and immunotherapy. While tumor-recruited CD11b+ myeloid cells are known mediators of tumor-associated immune dysfunction, the true nature of these suppressive cells and the fine biochemical pathways governing their immunosuppressive activity remain elusive. Here we describe a population of circulating CD11b+IL-4 receptor alpha+ (CD11b+IL-4Ralpha+), inflammatory-type monocytes that is elicited by growing tumors and activated by IFN-gamma released from T lymphocytes. CD11b+IL-4Ralpha+ cells produced IL-13 and IFN-gamma and integrated the downstream signals of these cytokines to trigger the molecular pathways suppressing antigen-activated CD8+ T lymphocytes. Analogous immunosuppressive circuits were active in CD11b+ cells present within the tumor microenvironment. These suppressor cells challenge the current idea that tumor-conditioned immunosuppressive monocytes/macrophages are alternatively activated. Moreover, our data show how the inflammatory response elicited by tumors had detrimental effects on the adaptive immune system and suggest novel approaches for the treatment of tumor-induced immune dysfunctions.
Figures


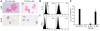

Comment in
-
Myeloid suppressor cells regulate the adaptive immune response to cancer.J Clin Invest. 2006 Oct;116(10):2587-90. doi: 10.1172/JCI29906. J Clin Invest. 2006. PMID: 17016554 Free PMC article.
Similar articles
-
CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer.Am J Respir Crit Care Med. 2012 Nov 15;186(10):1025-36. doi: 10.1164/rccm.201204-0636OC. Epub 2012 Sep 6. Am J Respir Crit Care Med. 2012. PMID: 22955317 Free PMC article.
-
Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14⁻/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer.J Cancer Res Clin Oncol. 2010 Jan;136(1):35-45. doi: 10.1007/s00432-009-0634-0. J Cancer Res Clin Oncol. 2010. PMID: 19572148
-
Myeloid suppressor cells regulate the adaptive immune response to cancer.J Clin Invest. 2006 Oct;116(10):2587-90. doi: 10.1172/JCI29906. J Clin Invest. 2006. PMID: 17016554 Free PMC article.
-
Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression.Cancer Immunol Immunother. 2005 Nov;54(11):1137-42. doi: 10.1007/s00262-005-0703-4. Epub 2005 May 5. Cancer Immunol Immunother. 2005. PMID: 15877228 Free PMC article. Review.
-
Immature myeloid cells and cancer-associated immune suppression.Cancer Immunol Immunother. 2002 Aug;51(6):293-8. doi: 10.1007/s00262-002-0280-8. Epub 2002 Apr 24. Cancer Immunol Immunother. 2002. PMID: 12111117 Free PMC article. Review.
Cited by
-
Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism.PLoS One. 2011;6(11):e27690. doi: 10.1371/journal.pone.0027690. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22110722 Free PMC article.
-
The antitumor activity of hPRDX5 against pancreatic cancer and the possible mechanisms.Braz J Med Biol Res. 2022 Sep 12;55:e12324. doi: 10.1590/1414-431X2022e12324. eCollection 2022. Braz J Med Biol Res. 2022. PMID: 36102418 Free PMC article.
-
Pembrolizumab in combination with gemcitabine for patients with HER2-negative advanced breast cancer: GEICAM/2015-04 (PANGEA-Breast) study.BMC Cancer. 2022 Dec 3;22(1):1258. doi: 10.1186/s12885-022-10363-3. BMC Cancer. 2022. PMID: 36463104 Free PMC article. Clinical Trial.
-
A DNA Hypomethylating Drug Alters the Tumor Microenvironment and Improves the Effectiveness of Immune Checkpoint Inhibitors in a Mouse Model of Pancreatic Cancer.Cancer Res. 2020 Nov 1;80(21):4754-4767. doi: 10.1158/0008-5472.CAN-20-0285. Epub 2020 Aug 14. Cancer Res. 2020. PMID: 32816859 Free PMC article.
-
Metabolic Plasticity of Neutrophils: Relevance to Pathogen Responses and Cancer.Trends Cancer. 2021 Aug;7(8):700-713. doi: 10.1016/j.trecan.2021.04.007. Epub 2021 May 19. Trends Cancer. 2021. PMID: 34023325 Free PMC article. Review.
References
-
- Kusmartsev S., Nefedova Y., Yoder D., Gabrilovich D.I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 2004;172:989–999. - PubMed
-
- Almand B., et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

