Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2
- PMID: 17006543
- PMCID: PMC1618114
- DOI: 10.1038/sj.emboj.7601351
Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2
Abstract
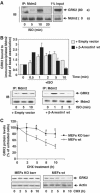
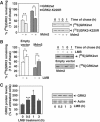
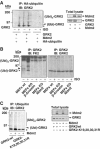
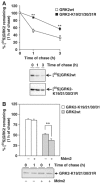
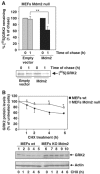
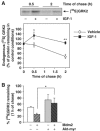
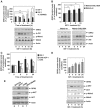
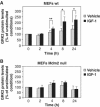
G-protein-coupled receptor kinase 2 (GRK2) is a central regulator of G-protein-coupled receptor signaling. We report that Mdm2, an E3-ubiquitin ligase involved in the control of cell growth and apoptosis, plays a key role in GRK2 degradation. Mdm2 and GRK2 association is enhanced by beta(2)-adrenergic receptor stimulation and beta-arrestin. Increased Mdm2 expression accelerates GRK2 proteolysis and promotes kinase ubiquitination at defined residues, whereas GRK2 turnover is markedly impaired in Mdm2-deficient cells. Moreover, we find that activation of the PI3K/Akt pathway by insulin-like growth factor-1 alters Mdm2-mediated GRK2 degradation, leading to enhanced GRK2 stability and increased kinase levels. These data put forward a novel mechanism for controlling GRK2 expression in physiological and pathological conditions.
Figures
Similar articles
-
Multiple scaffolding functions of {beta}-arrestins in the degradation of G protein-coupled receptor kinase 2.J Biol Chem. 2011 Jan 14;286(2):1165-73. doi: 10.1074/jbc.M110.203406. Epub 2010 Nov 16. J Biol Chem. 2011. PMID: 21081496 Free PMC article.
-
GRK2-mediated receptor phosphorylation and Mdm2-mediated β-arrestin2 ubiquitination drive clathrin-mediated endocytosis of G protein-coupled receptors.Biochem Biophys Res Commun. 2020 Dec 10;533(3):383-390. doi: 10.1016/j.bbrc.2020.09.030. Epub 2020 Sep 19. Biochem Biophys Res Commun. 2020. PMID: 32962859
-
Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2.EMBO J. 2001 Sep 17;20(18):5129-38. doi: 10.1093/emboj/20.18.5129. EMBO J. 2001. PMID: 11566877 Free PMC article.
-
Chapter Three - Ubiquitination and Protein Turnover of G-Protein-Coupled Receptor Kinases in GPCR Signaling and Cellular Regulation.Prog Mol Biol Transl Sci. 2016;141:85-140. doi: 10.1016/bs.pmbts.2016.04.002. Epub 2016 May 7. Prog Mol Biol Transl Sci. 2016. PMID: 27378756 Review.
-
Beta-arrestin and Mdm2, unsuspected partners in signaling from the cell surface.Sci STKE. 2001 Nov 27;2001(110):pe41. doi: 10.1126/stke.2001.110.pe41. Sci STKE. 2001. PMID: 11724970 Review.
Cited by
-
The Ubiquitination of Arrestin3 within the Nucleus Triggers the Nuclear Export of Mdm2, Which, in Turn, Mediates the Ubiquitination of GRK2 in the Cytosol.Int J Mol Sci. 2024 Sep 6;25(17):9644. doi: 10.3390/ijms25179644. Int J Mol Sci. 2024. PMID: 39273591 Free PMC article.
-
A common molecular and cellular pathway in developing Alzheimer and cancer.Biochem Biophys Rep. 2023 Dec 26;37:101625. doi: 10.1016/j.bbrep.2023.101625. eCollection 2024 Mar. Biochem Biophys Rep. 2023. PMID: 38225990 Free PMC article. Review.
-
β-adrenergic receptor signaling mediated by β-arrestins and its potential role in heart failure.Curr Opin Physiol. 2024 Feb;37:100723. doi: 10.1016/j.cophys.2023.100723. Epub 2023 Nov 18. Curr Opin Physiol. 2024. PMID: 38094036
-
Ubiquitination of GRK2 Is Required for the β-Arrestin-Biased Signaling Pathway of Dopamine D2 Receptors to Activate ERK Kinases.Int J Mol Sci. 2023 Jun 12;24(12):10031. doi: 10.3390/ijms241210031. Int J Mol Sci. 2023. PMID: 37373182 Free PMC article.
-
Myocardial Protection and Current Cancer Therapy: Two Opposite Targets with Inevitable Cost.Int J Mol Sci. 2022 Nov 15;23(22):14121. doi: 10.3390/ijms232214121. Int J Mol Sci. 2022. PMID: 36430599 Free PMC article. Review.
References
-
- Ashcroft M, Ludwig RL, Woods DB, Copeland TD, Weber HO, MacRae EJ, Vousden KH (2002) Phosphorylation of HDM2 by Akt. Oncogene 21: 1955–1962 - PubMed
-
- Bond GL, Hu W, Levine AJ (2005) MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets 5: 3–8 - PubMed
-
- Elorza A, Penela P, Sarnago S, Mayor F Jr (2003) MAPK-dependent degradation of G protein-coupled receptor kinase 2. J Biol Chem 278: 29164–29173 - PubMed
-
- Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, Hemmings BA (2004) Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem 279: 35510–35517 - PubMed
-
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

