Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins
- PMID: 17005686
- PMCID: PMC1617298
- DOI: 10.1128/JVI.00607-06
Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins
Abstract
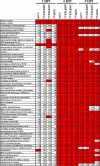
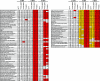
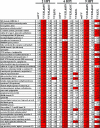
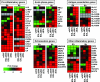
Cells have evolved elaborate mechanisms to counteract the onslaught of viral infections. To activate these defenses, the viral threat must be recognized. Danger signals, or pathogen-associated molecular patterns, that are induced by pathogens include double-stranded RNA (dsRNA), viral single-stranded RNA, glycolipids, and CpG DNA. Understanding the signal transduction pathways activated and host gene expression induced by these danger signals is vital to understanding virus-host interactions. The vaccinia virus E3L protein is involved in blocking the host antiviral response and increasing pathogenesis, functions that map to separate C-terminal dsRNA- and N-terminal Z-DNA-binding domains. Viruses containing mutations in these domains allow modeling of the role of dsRNA and Z-form nucleic acid in the host response to virus infection. Deletions in the Z-DNA- or dsRNA-binding domains led to activation of signal transduction cascades and up-regulation of host gene expression, with many genes involved in the inflammatory response. These data suggest that poxviruses actively inhibit cellular recognition of viral danger signals and the subsequent cellular response to the viral threat.
Figures


Similar articles
-
Mutational analysis of vaccinia virus E3 protein: the biological functions do not correlate with its biochemical capacity to bind double-stranded RNA.J Virol. 2015 May;89(10):5382-94. doi: 10.1128/JVI.03288-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740987 Free PMC article.
-
Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells.Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12759-64. doi: 10.1073/pnas.0506011102. Epub 2005 Aug 26. Proc Natl Acad Sci U S A. 2005. PMID: 16126896 Free PMC article.
-
Role of viral factor E3L in modified vaccinia virus ankara infection of human HeLa Cells: regulation of the virus life cycle and identification of differentially expressed host genes.J Virol. 2005 Feb;79(4):2584-96. doi: 10.1128/JVI.79.4.2584-2596.2005. J Virol. 2005. PMID: 15681458 Free PMC article.
-
The dsRNA protein kinase PKR: virus and cell control.Biochimie. 2007 Jun-Jul;89(6-7):799-811. doi: 10.1016/j.biochi.2007.03.001. Epub 2007 Mar 12. Biochimie. 2007. PMID: 17451862 Review.
-
How vaccinia virus has evolved to subvert the host immune response.J Struct Biol. 2011 Aug;175(2):127-34. doi: 10.1016/j.jsb.2011.03.010. Epub 2011 Mar 17. J Struct Biol. 2011. PMID: 21419849 Free PMC article. Review.
Cited by
-
Poxviral protein E3-altered cytokine production reveals that DExD/H-box helicase 9 controls Toll-like receptor-stimulated immune responses.J Biol Chem. 2018 Sep 28;293(39):14989-15001. doi: 10.1074/jbc.RA118.005089. Epub 2018 Aug 15. J Biol Chem. 2018. PMID: 30111593 Free PMC article.
-
Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling.J Biol Chem. 2008 Dec 12;283(50):34580-7. doi: 10.1074/jbc.M807029200. Epub 2008 Oct 15. J Biol Chem. 2008. PMID: 18927075 Free PMC article.
-
Vaccinia virus F1L interacts with Bak using highly divergent Bcl-2 homology domains and replaces the function of Mcl-1.J Biol Chem. 2010 Feb 12;285(7):4695-708. doi: 10.1074/jbc.M109.053769. Epub 2009 Dec 2. J Biol Chem. 2010. PMID: 19955184 Free PMC article.
-
Attenuated NYCBH vaccinia virus deleted for the E3L gene confers partial protection against lethal monkeypox virus disease in cynomolgus macaques.Vaccine. 2011 Dec 6;29(52):9684-90. doi: 10.1016/j.vaccine.2011.09.135. Epub 2011 Oct 12. Vaccine. 2011. PMID: 22001879 Free PMC article.
-
Human Cytomegalovirus pTRS1 and pIRS1 Antagonize Protein Kinase R To Facilitate Virus Replication.J Virol. 2016 Mar 28;90(8):3839-3848. doi: 10.1128/JVI.02714-15. Print 2016 Apr. J Virol. 2016. PMID: 26819306 Free PMC article.
References
-
- Baylis, C. D., and R. C. Condit. 1993. Temperature-sensitive mutants in the vaccinia virus A18R gene increase double-stranded RNA synthesis as a result of aberrant viral transcription. Virology 194:254-262. - PubMed
-
- Beattie, E., E. B. Kaufman, H. Marinez, M. E. Perus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. - PubMed
-
- Blanchard, T. J., P. Alcami, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. - PubMed
-
- Brandt, T., M. Heck, A. Vijaysri, G. Jentarra, J. Cameron, and B. Jacobs. 2005. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not for induction of a protective immune response. Virology 333:263-270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI 022646-20A/AI/NIAID NIH HHS/United States
- AI 056214-02/AI/NIAID NIH HHS/United States
- DA 015623-03/DA/NIDA NIH HHS/United States
- R21 AI052347/AI/NIAID NIH HHS/United States
- P30 DA015625/DA/NIDA NIH HHS/United States
- R01 AI052347/AI/NIAID NIH HHS/United States
- U01 AI066326/AI/NIAID NIH HHS/United States
- R01 AI022646/AI/NIAID NIH HHS/United States
- R01 AI066501/AI/NIAID NIH HHS/United States
- R21 AI056214/AI/NIAID NIH HHS/United States
- AI 066326/AI/NIAID NIH HHS/United States
- AI 066501/AI/NIAID NIH HHS/United States
- AI 052347/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

