Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies
- PMID: 17005652
- PMCID: PMC1676306
- DOI: 10.1128/JVI.01348-06
Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies
Abstract
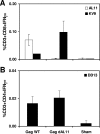
Generating broad cellular immune responses against a diversity of viral epitopes is a major goal of current vaccine strategies for human immunodeficiency virus type 1 (HIV-1) and other pathogens. Virus-specific CD8(+) T-lymphocyte responses, however, are often highly focused on a very limited number of immunodominant epitopes. For an HIV-1 vaccine, the breadth of CD8(+) T-lymphocyte responses may prove to be critical as a result of the need to cover a wide diversity of viral isolates in the population and to limit viral escape from dominant epitope-specific T lymphocytes. Here we show that epitope modification strategies can alter CD8(+) T-lymphocyte epitope immunodominance hierarchies elicited by a DNA vaccine in mice. Mice immunized with a DNA vaccine expressing simian immunodeficiency virus Gag lacking the dominant D(b)-restricted AL11 epitope generated a marked and durable augmentation of responses specific for the subdominant D(b)-restricted KV9 epitope. Moreover, anatomic separation strategies and heterologous prime-boost regimens generated codominant responses against both epitopes. These data demonstrate that dominant epitopes can dramatically suppress the immunogenicity of subdominant epitopes in the context of gene-based vaccines and that epitope modification strategies can be utilized to enhance responses to subdominant epitopes.
Figures


Similar articles
-
A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (SIV) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in Mamu-A*01 rhesus macaques.J Immunol. 2003 Apr 15;170(8):4281-9. doi: 10.4049/jimmunol.170.8.4281. J Immunol. 2003. PMID: 12682263
-
Silencing an immunodominant epitope of hepatitis B surface antigen reveals an alternative repertoire of CD8 T cell epitopes of this viral antigen.Vaccine. 2009 Dec 10;28(1):114-9. doi: 10.1016/j.vaccine.2009.09.096. Epub 2009 Oct 8. Vaccine. 2009. PMID: 19818719
-
Recombinant canarypox vaccine-elicited CTL specific for dominant and subdominant simian immunodeficiency virus epitopes in rhesus monkeys.J Immunol. 2002 Feb 15;168(4):1847-53. doi: 10.4049/jimmunol.168.4.1847. J Immunol. 2002. PMID: 11823518
-
Revealing factors determining immunodominant responses against dominant epitopes.Immunogenetics. 2020 Feb;72(1-2):109-118. doi: 10.1007/s00251-019-01134-9. Epub 2019 Dec 6. Immunogenetics. 2020. PMID: 31811313 Free PMC article. Review.
-
Identification and selection of immunodominant B and T cell epitopes for dengue multi-epitope-based vaccine.Med Microbiol Immunol. 2021 Feb;210(1):1-11. doi: 10.1007/s00430-021-00700-x. Epub 2021 Jan 30. Med Microbiol Immunol. 2021. PMID: 33515283 Review.
Cited by
-
Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection.J Control Release. 2013 Sep 28;170(3):452-9. doi: 10.1016/j.jconrel.2013.06.004. Epub 2013 Jun 14. J Control Release. 2013. PMID: 23774102 Free PMC article.
-
CD8+ T-cell cross-competition is governed by peptide-MHC class I stability.Eur J Immunol. 2012 Jan;42(1):256-63. doi: 10.1002/eji.201142010. Epub 2011 Nov 28. Eur J Immunol. 2012. PMID: 22002320 Free PMC article.
-
Bioinformatics for cancer immunotherapy target discovery.Cancer Immunol Immunother. 2014 Dec;63(12):1235-49. doi: 10.1007/s00262-014-1627-7. Epub 2014 Oct 26. Cancer Immunol Immunother. 2014. PMID: 25344903 Free PMC article. Review.
-
Increased Valency of Conserved-mosaic Vaccines Enhances the Breadth and Depth of Epitope Recognition.Mol Ther. 2016 Feb;24(2):375-384. doi: 10.1038/mt.2015.210. Epub 2015 Nov 19. Mol Ther. 2016. PMID: 26581160 Free PMC article.
-
Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination.J Immunol. 2008 Sep 15;181(6):4188-98. doi: 10.4049/jimmunol.181.6.4188. J Immunol. 2008. PMID: 18768876 Free PMC article.
References
-
- Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. - PubMed
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Barouch, D. H., A. Craiu, S. Santra, M. A. Egan, J. E. Schmitz, M. J. Kuroda, T. M. Fu, J. H. Nam, L. S. Wyatt, M. A. Lifton, G. R. Krivulka, C. E. Nickerson, C. I. Lord, B. Moss, M. G. Lewis, V. M. Hirsch, J. W. Shiver, and N. L. Letvin. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462-2467. - PMC - PubMed
-
- Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. - PubMed
-
- Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

