Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation
- PMID: 17003115
- PMCID: PMC1595452
- DOI: 10.1073/pnas.0606805103
Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation
Abstract
Declining estrogen production after menopause causes osteoporosis in which the resorption of bone exceeds the increase in bone formation. We recently found that mice deficient in the beta-subunit of follicle-stimulating hormone (FSHbeta) are protected from bone loss despite severe estrogen deficiency. Here we show that FSHbeta-deficient mice have lowered TNFalpha levels. However, TNFalpha-deficient mice are resistant to hypogonadal bone loss despite having elevated FSH, suggesting that TNFalpha is critical to the effect of FSH on bone mass. We find that FSH directly stimulates TNFalpha production from bone marrow granulocytes and macrophages. We also explore how TNFalpha up-regulation induces bone loss. By modeling the known actions of TNFalpha, we attribute the high-turnover bone loss to an expanded osteoclast precursor pool, together with enhanced osteoblast formation. TNFalpha inhibits osteoblastogenesis in the presence of ascorbic acid in culture medium, but in its absence this effect becomes stimulatory; thus, ascorbic acid reverses the true action of TNFalpha. Likewise, ascorbic acid blunts the effects of TNFalpha in stimulating osteoclast formation. We propose that hypogonadal bone loss is caused, at least in part, by enhanced FSH secretion, which in turn increases TNFalpha production to expand the number of bone marrow osteoclast precursors. Ascorbic acid may prevent FSH-induced hypogonadal bone loss by modulating the catabolic actions of TNFalpha.
Conflict of interest statement
Cnflict of interest statement: M.Z. is on the speaker bureau for Roche, GlaxoSmithKline, Aventis, Procter & Gamble, and Merck. M.Z. has research grant support from Procter & Gamble.
Figures
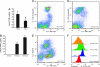
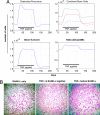
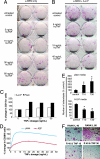
Similar articles
-
Tumor necrosis factor-alpha: alternative role as an inhibitor of osteoclast formation in vitro.Bone. 2006 Aug;39(2):325-35. doi: 10.1016/j.bone.2006.02.056. Epub 2006 Mar 31. Bone. 2006. PMID: 16580896
-
Cytokine-induced nitric oxide inhibits bone resorption by inducing apoptosis of osteoclast progenitors and suppressing osteoclast activity.J Bone Miner Res. 1997 Nov;12(11):1797-804. doi: 10.1359/jbmr.1997.12.11.1797. J Bone Miner Res. 1997. PMID: 9383684
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Interleukin-33, a target of parathyroid hormone and oncostatin m, increases osteoblastic matrix mineral deposition and inhibits osteoclast formation in vitro.Endocrinology. 2011 May;152(5):1911-22. doi: 10.1210/en.2010-1268. Epub 2011 Mar 1. Endocrinology. 2011. PMID: 21363931
-
The Relationship between Follicle-stimulating Hormone and Bone Health: Alternative Explanation for Bone Loss beyond Oestrogen?Int J Med Sci. 2018 Sep 7;15(12):1373-1383. doi: 10.7150/ijms.26571. eCollection 2018. Int J Med Sci. 2018. PMID: 30275766 Free PMC article. Review.
Cited by
-
Independent Skeletal Actions of Pituitary Hormones.Endocrinol Metab (Seoul). 2022 Oct;37(5):719-731. doi: 10.3803/EnM.2022.1573. Epub 2022 Sep 28. Endocrinol Metab (Seoul). 2022. PMID: 36168775 Free PMC article.
-
From the gut to the strut: where inflammation reigns, bone abstains.J Clin Invest. 2016 Jun 1;126(6):2045-8. doi: 10.1172/JCI87430. Epub 2016 Apr 25. J Clin Invest. 2016. PMID: 27111233 Free PMC article.
-
FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts.Biochem Biophys Res Commun. 2010 Mar 26;394(1):12-7. doi: 10.1016/j.bbrc.2010.02.112. Epub 2010 Feb 19. Biochem Biophys Res Commun. 2010. PMID: 20171950 Free PMC article.
-
Bone Fragility in Turner Syndrome: Mechanisms and Prevention Strategies.Front Endocrinol (Lausanne). 2016 Apr 26;7:34. doi: 10.3389/fendo.2016.00034. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27199891 Free PMC article. Review.
-
Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice.Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5229-34. doi: 10.1073/pnas.0608557104. Epub 2007 Mar 8. Proc Natl Acad Sci U S A. 2007. PMID: 17360389 Free PMC article.
References
-
- US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004. pp. 68–87.
-
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, et al. Cell. 2006;125:247–260. - PubMed
-
- van der Poll T, Romijn JA, Endert E, Sauerwein HP. Metabolism. 1993;42:303–307. - PubMed
-
- Hutson JC. J Reprod Immunol. 1993;23:63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

