Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse
- PMID: 16995882
- PMCID: PMC2265821
- DOI: 10.1111/j.1365-2567.2006.02458.x
Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse
Abstract
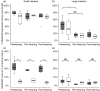
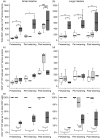
Colonization with commensal flora in very early life may profoundly influence intestinal lymphoid development and bias later immune responses. We defined gut-homing T cell phenotypes and the influence of flora on intestinal immune development in mice. Intestinal T cells were phenotyped and quantified in conventional (CV), germfree (GF) and conventionalized germfree (GF/CV) neonatal mice by immunohistochemistry. Mucosal adressin cell adhesion molecule 1 (MAdCAM-1) was expressed by mucosal vessels at birth in CV and GF mice and was more prevalent in CV than GF small intestine, but was distributed similarly and did not change with age. Less MAdCAM-1 was expressed in the colon; its distribution became restricted after weaning, with no difference between CV and GF mice. CD3(+)beta(7) (+) cells were present in similar numbers in CV and GF intestine at birth. They were CD62L(-) in CV mice and were accompanied by further CD3(+)beta(7) (+)CD62L(-) T cells as development progressed, but in GF and GF/CV intestine they expressed CD62L and numbers did not change. IEL numbers increased at weaning in CV mice in both small and large intestine, but showed delayed development in GF intestine. Macrophages were present at high levels from birth in GF intestine, but dendritic cells did not develop until day 16. Thus, fetus-derived T cells seed the intestinal lamina propria before birth via beta-MadCAM interactions. Their activation status depends on the microbiological status of the dam, and without a commensal flora they remain naive. We propose that these cells regulate antigen responsiveness of the developing mucosal T cell pool.
Figures

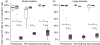
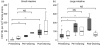

Similar articles
-
Differences in development of lymphocyte subpopulations from gut-associated lymphatic tissue (GALT) of germfree and conventional rats: effect of aging.Folia Microbiol (Praha). 1998;43(5):531-4. doi: 10.1007/BF02820814. Folia Microbiol (Praha). 1998. PMID: 9821320
-
Age-related changes in the distribution and frequency of myeloid and T cell populations in the small intestine of calves.Cell Immunol. 2011;271(2):428-37. doi: 10.1016/j.cellimm.2011.08.012. Epub 2011 Aug 23. Cell Immunol. 2011. PMID: 21917242
-
Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system.Infect Immun. 1999 Jul;67(7):3504-11. doi: 10.1128/IAI.67.7.3504-3511.1999. Infect Immun. 1999. PMID: 10377132 Free PMC article.
-
Generation of gut-homing T cells and their localization to the small intestinal mucosa.Immunol Rev. 2007 Feb;215:226-42. doi: 10.1111/j.1600-065X.2006.00482.x. Immunol Rev. 2007. PMID: 17291292 Review.
-
Compartmentalization of the mucosal immune responses to commensal intestinal bacteria.Ann N Y Acad Sci. 2004 Dec;1029:36-43. doi: 10.1196/annals.1309.005. Ann N Y Acad Sci. 2004. PMID: 15681741 Review.
Cited by
-
Multi-omics analysis reveals the effects of microbiota on oral homeostasis.Front Immunol. 2022 Sep 20;13:1005992. doi: 10.3389/fimmu.2022.1005992. eCollection 2022. Front Immunol. 2022. PMID: 36211346 Free PMC article.
-
Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology.Nutrients. 2013 May 29;5(6):1869-912. doi: 10.3390/nu5061869. Nutrients. 2013. PMID: 23760057 Free PMC article. Review.
-
Modulation of Gut Microbiota: A Novel Paradigm of Enhancing the Efficacy of Programmed Death-1 and Programmed Death Ligand-1 Blockade Therapy.Front Immunol. 2018 Mar 5;9:374. doi: 10.3389/fimmu.2018.00374. eCollection 2018. Front Immunol. 2018. PMID: 29556232 Free PMC article. Review.
-
Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates.Mar Drugs. 2023 Feb 23;21(3):144. doi: 10.3390/md21030144. Mar Drugs. 2023. PMID: 36976193 Free PMC article. Review.
-
Immunopathogenesis and therapeutic approaches in pediatric celiac disease.Expert Rev Clin Immunol. 2016 Aug;12(8):857-69. doi: 10.1586/1744666X.2016.1168294. Epub 2016 Apr 1. Expert Rev Clin Immunol. 2016. PMID: 26999328 Free PMC article. Review.
References
-
- Howie D, Spencer J, deLord D, et al. Extrathymic T cell differentiation in the human intestine early in life. J Immunol. 1998;161:5862–72. - PubMed
-
- Gunther U, Holloway JA, Gordon JG, et al. Phenotypic characterization of CD3-7+ cells in developing human intestine and an analysis of their ability to differentiate into T cells. J Immunol. 2005;174:5414–22. - PubMed
-
- Williams AM, Bland PW, Phillips AC, et al. Intestinal αβ T cells differentiate and rearrange antigen receptor genes in situ in the human infant. J Immunol. 2004;173:7190–9. - PubMed
-
- Adachi S, Yoshida H, Kataoka H, et al. Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol. 1997;9:507–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

