PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation
- PMID: 16990793
- PMCID: PMC1589987
- DOI: 10.1038/sj.emboj.7601346
PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation
Abstract
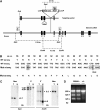
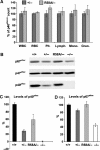
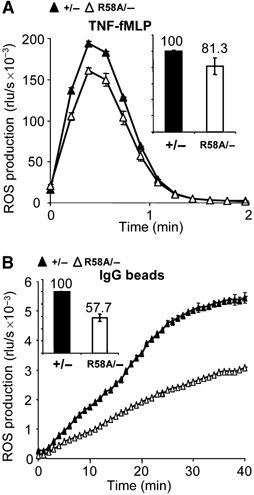
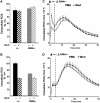
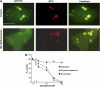
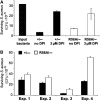
The production of reactive oxygen species by the NADPH oxidase complex of phagocytes plays a critical role in our defence against bacterial and fungal infections. The PX domains of two oxidase components, p47(phox) and p40(phox), are known to bind phosphoinositide products of PI3Ks but the physiological roles of these interactions are unclear. We have created mice which carry an R58A mutation in the PX domain of their p40(phox) gene, which selectively prevents binding to PtdIns3P. p40(phoxR58A/R58A) embryos do not develop normally but p40(phoxR58A/-) mice are viable and neutrophils from these animals exhibit significantly reduced oxidase responses compared to those from their p40(phox+/-) siblings (e.g. 60% reduced in response to phagocytosis of Staphylococcus aureus). Wortmannin inhibition of the S. aureus oxidase response correlates with inhibition of phagosomal PtdIns3P accumulation and overlaps with the reduction in this response caused by the R58A mutation, suggesting PI3K regulation of this response is substantially dependent on PtdIns3P-binding to p40(phox). p40(phoxR58A/-) mice are significantly compromised in their ability to kill S. aureus in vivo, defining the physiological importance of this interaction.
Figures
Similar articles
-
Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing.J Exp Med. 2006 Aug 7;203(8):1927-37. doi: 10.1084/jem.20052069. Epub 2006 Jul 31. J Exp Med. 2006. PMID: 16880254 Free PMC article.
-
Cooperation of p40(phox) with p47(phox) for Nox2-based NADPH oxidase activation during Fcγ receptor (FcγR)-mediated phagocytosis: mechanism for acquisition of p40(phox) phosphatidylinositol 3-phosphate (PI(3)P) binding.J Biol Chem. 2011 Nov 25;286(47):40693-705. doi: 10.1074/jbc.M111.237289. Epub 2011 Sep 28. J Biol Chem. 2011. PMID: 21956105 Free PMC article.
-
Phosphorylation of threonine 154 in p40phox is an important physiological signal for activation of the neutrophil NADPH oxidase.Blood. 2010 Dec 23;116(26):6027-36. doi: 10.1182/blood-2010-08-300889. Epub 2010 Sep 22. Blood. 2010. PMID: 20861461 Free PMC article.
-
NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits.Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12951. doi: 10.1111/eci.12951. Epub 2018 Jun 3. Eur J Clin Invest. 2018. PMID: 29757466 Review.
-
The role of PI3Ks in the regulation of the neutrophil NADPH oxidase.Biochem Soc Symp. 2007;(74):59-67. doi: 10.1042/BSS0740059. Biochem Soc Symp. 2007. PMID: 17233580 Review.
Cited by
-
Role of p85α in neutrophil extra- and intracellular reactive oxygen species generation.Oncotarget. 2016 Apr 26;7(17):23096-105. doi: 10.18632/oncotarget.8500. Oncotarget. 2016. PMID: 27049833 Free PMC article.
-
The emerging mechanisms of isoform-specific PI3K signalling.Nat Rev Mol Cell Biol. 2010 May;11(5):329-41. doi: 10.1038/nrm2882. Epub 2010 Apr 9. Nat Rev Mol Cell Biol. 2010. PMID: 20379207 Review.
-
PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils.Blood. 2010 Dec 2;116(23):4978-89. doi: 10.1182/blood-2010-03-275602. Epub 2010 Sep 2. Blood. 2010. PMID: 20813901 Free PMC article.
-
Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function.Front Cell Dev Biol. 2022 Jul 14;10:945749. doi: 10.3389/fcell.2022.945749. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912108 Free PMC article. Review.
-
The Macroautophagy Machinery in MHC Restricted Antigen Presentation.Front Immunol. 2021 Feb 25;12:628429. doi: 10.3389/fimmu.2021.628429. eCollection 2021. Front Immunol. 2021. PMID: 33717153 Free PMC article. Review.
References
-
- Ago T, Takeya R, Hiroaki H, Kuribayashi F, Ito T, Kohda D, Sumimoto H (2001) The PX domain as a novel phosphoinositide-binding molecule. Biochem Biophys Res Commun 287: 733–738 - PubMed
-
- Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK (2004) Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173: 5730–5738 - PubMed
-
- Bravo J, Karathanassis D, Pacold CM, Pacold ME, Ellson CD, Anderson KE, Butler PJ, Lavenir I, Perisic O, Hawkins PT, Stephens L, Williams RL (2001) The crystal structure of the PX domain of p40Phox bound to phosphatidylinositiol 3-phosphate. Mol Cell 8: 829–839 - PubMed
-
- Brown GE, Stewart MQ, Liu H, Ha VL, Yaffe MB (2003) A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell 11: 35–47 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

