Identification of Sox17 as a transcription factor that regulates oligodendrocyte development
- PMID: 16988043
- PMCID: PMC6674459
- DOI: 10.1523/JNEUROSCI.1716-06.2006
Identification of Sox17 as a transcription factor that regulates oligodendrocyte development
Abstract
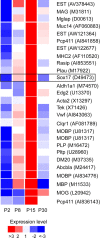
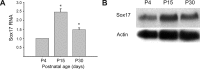
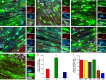
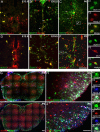
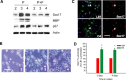
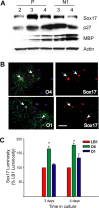
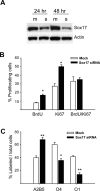
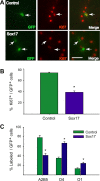
Microarray analysis of oligodendrocyte lineage cells purified by fluorescence-activated cell sorting (FACS) from 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNP)-enhanced green fluorescent protein (EGFP) transgenic mice revealed Sox17 (SRY-box containing gene 17) gene expression to be coordinately regulated with that of four myelin genes during postnatal development. In CNP-EGFP-positive (CNP-EGFP+) cells, Sox17 mRNA and protein levels transiently increased between postnatal days 2 and 15, with white matter O4+ preoligodendrocytes expressing greater Sox17 levels than Nkx2.2+ (NK2 transcription factor related, locus 2) NG2+, or GalC+ (galactocerebroside) cells. In spinal cord, Sox17 protein expression was undetectable in the primary motor neuron domain between embryonic days 12.5 and 15.5 but was evident in Nkx2.2+ and CC1+ cells. In cultured oligodendrocyte progenitor cells (OPCs), Sox17 levels were maximal in O4+ cells and peaked during the phenotypic conversion from bipolar to multipolar. Parallel increases in Sox17 and p27 occurred before MBP protein expression, and Sox17 upregulation was prevented by conditions inhibiting differentiation. Sox17 downregulation with small interfering RNAs increased OPC proliferation and decreased lineage progression after mitogen withdrawal, whereas Sox17 overexpression in the presence of mitogen had opposite effects. Sox17 overexpression enhanced myelin gene expression in OPCs and directly stimulated MBP gene promoter activity. These findings support important roles for Sox17 in controlling both oligodendrocyte progenitor cell cycle exit and differentiation.
Figures

Similar articles
-
SRY-box containing gene 17 regulates the Wnt/β-catenin signaling pathway in oligodendrocyte progenitor cells.J Neurosci. 2011 Sep 28;31(39):13921-35. doi: 10.1523/JNEUROSCI.3343-11.2011. J Neurosci. 2011. PMID: 21957254 Free PMC article.
-
Transgenic overexpression of Sox17 promotes oligodendrocyte development and attenuates demyelination.J Neurosci. 2013 Jul 24;33(30):12528-42. doi: 10.1523/JNEUROSCI.0536-13.2013. J Neurosci. 2013. PMID: 23884956 Free PMC article.
-
SOX17 transcription factor negatively regulates oligodendrocyte precursor cell differentiation.Glia. 2018 Oct;66(10):2221-2232. doi: 10.1002/glia.23483. Epub 2018 Aug 26. Glia. 2018. PMID: 30152028
-
Timing cell-cycle exit and differentiation in oligodendrocyte development.Novartis Found Symp. 2001;237:100-7; discussion 107-12, 158-63. doi: 10.1002/0470846666.ch9. Novartis Found Symp. 2001. PMID: 11444039 Review.
-
Sry-related High Mobility Group Box 17 Functions as a Tumor Suppressor by Antagonizing the Wingless-related Integration Site Pathway.J Cancer Prev. 2020 Dec 30;25(4):204-212. doi: 10.15430/JCP.2020.25.4.204. J Cancer Prev. 2020. PMID: 33409253 Free PMC article. Review.
Cited by
-
Tumor-initiating label-retaining cancer cells in human gastrointestinal cancers undergo asymmetric cell division.Stem Cells. 2012 Apr;30(4):591-8. doi: 10.1002/stem.1061. Stem Cells. 2012. PMID: 22331764 Free PMC article.
-
Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease.J Mol Neurosci. 2008 May;35(1):13-22. doi: 10.1007/s12031-007-9014-x. J Mol Neurosci. 2008. PMID: 17999198 Free PMC article. Review.
-
SOX7 expression is critically required in FLK1-expressing cells for vasculogenesis and angiogenesis during mouse embryonic development.Mech Dev. 2017 Aug;146:31-41. doi: 10.1016/j.mod.2017.05.004. Epub 2017 May 31. Mech Dev. 2017. PMID: 28577909 Free PMC article.
-
Sox17 promoter methylation in plasma DNA is associated with poor survival and can be used as a prognostic factor in breast cancer.Medicine (Baltimore). 2015 Mar;94(11):e637. doi: 10.1097/MD.0000000000000637. Medicine (Baltimore). 2015. PMID: 25789956 Free PMC article.
-
Two-tier transcriptional control of oligodendrocyte differentiation.Curr Opin Neurobiol. 2009 Oct;19(5):479-85. doi: 10.1016/j.conb.2009.08.004. Epub 2009 Sep 7. Curr Opin Neurobiol. 2009. PMID: 19740649 Free PMC article. Review.
References
-
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
-
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
