Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini
- PMID: 16987972
- PMCID: PMC1642591
- DOI: 10.1128/JVI.00857-06
Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini
Abstract
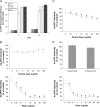
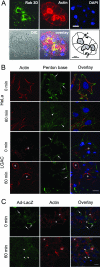
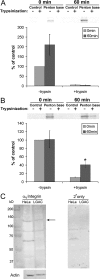
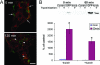
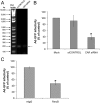
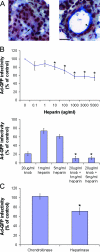
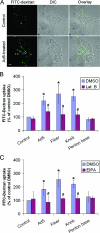
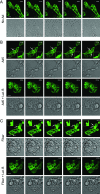
The established mechanism for infection of most cells with adenovirus serotype 5 (Ad5) involves fiber capsid protein binding to coxsackievirus-adenovirus receptor (CAR) at the cell surface, followed by penton base capsid protein binding to alpha(v) integrins, which triggers clathrin-mediated endocytosis of the virus. Here we determined the identity of the capsid proteins responsible for mediating Ad5 entry into the acinar epithelial cells of the lacrimal gland. Ad5 transduction of primary rabbit lacrimal acinar cells was inhibited by excess Ad5 fiber or knob (terminal region of the fiber) but not excess penton base. Investigation of the interactions of recombinant Ad5 penton base, fiber, and knob with lacrimal acini revealed that the penton base capsid protein remained surface associated, while the knob domain of the fiber capsid protein was rapidly internalized. Introduction of rabbit CAR-specific small interfering RNA (siRNA) into lacrimal acini under conditions that reduced intracellular CAR mRNA significantly inhibited Ad5 transduction, in contrast to a control (nonspecific) siRNA. Preincubation of Ad5 with excess heparin or pretreatment of acini with a heparinase cocktail each inhibited Ad5 transduction by a separate and apparently additive mechanism. Functional and imaging studies revealed that Ad5, fiber, and knob, but not penton base, stimulated macropinocytosis in acini and that inhibition of macropinocytosis significantly reduced Ad5 transduction of acini. However, inhibition of macropinocytosis did not reduce Ad5 uptake. We propose that internalization of Ad5 into lacrimal acini is through a novel fiber-dependent mechanism that includes CAR and heparan sulfate glycosaminoglycans and that the subsequent intracellular trafficking of Ad5 is enhanced by fiber-induced macropinocytosis.
Figures


Similar articles
-
The Ad5 fiber mediates nonviral gene transfer in the absence of the whole virus, utilizing a novel cell entry pathway.Gene Ther. 2005 Feb;12(3):225-37. doi: 10.1038/sj.gt.3302402. Gene Ther. 2005. PMID: 15483666
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
-
A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway.Clin Cancer Res. 2007 May 1;13(9):2777-83. doi: 10.1158/1078-0432.CCR-06-2706. Clin Cancer Res. 2007. PMID: 17473211 Free PMC article.
-
Dependence of adenovirus infectivity on length of the fiber shaft domain.J Virol. 2000 Nov;74(22):10274-86. doi: 10.1128/jvi.74.22.10274-10286.2000. J Virol. 2000. PMID: 11044071 Free PMC article.
-
Adenovirus endocytosis.J Gene Med. 2004 Feb;6 Suppl 1:S152-63. doi: 10.1002/jgm.553. J Gene Med. 2004. PMID: 14978758 Review.
Cited by
-
Internalization mechanisms of brain-derived tau oligomers from patients with Alzheimer's disease, progressive supranuclear palsy and dementia with Lewy bodies.Cell Death Dis. 2020 May 4;11(5):314. doi: 10.1038/s41419-020-2503-3. Cell Death Dis. 2020. PMID: 32366836 Free PMC article.
-
Rapid and efficient clearance of blood-borne virus by liver sinusoidal endothelium.PLoS Pathog. 2011 Sep;7(9):e1002281. doi: 10.1371/journal.ppat.1002281. Epub 2011 Sep 29. PLoS Pathog. 2011. PMID: 21980295 Free PMC article.
-
Development of adenovirus capsid proteins for targeted therapeutic delivery.Ther Deliv. 2013 Feb;4(2):267-77. doi: 10.4155/tde.12.155. Ther Deliv. 2013. PMID: 23343164 Free PMC article. Review.
-
Disruption of the coxsackievirus and adenovirus receptor-homodimeric interaction triggers lipid microdomain- and dynamin-dependent endocytosis and lysosomal targeting.J Biol Chem. 2014 Jan 10;289(2):680-95. doi: 10.1074/jbc.M113.518365. Epub 2013 Nov 22. J Biol Chem. 2014. PMID: 24273169 Free PMC article.
-
Adenovirus.Curr Top Microbiol Immunol. 2010;343:195-224. doi: 10.1007/82_2010_16. Curr Top Microbiol Immunol. 2010. PMID: 20376613 Free PMC article. Review.
References
-
- Baluska, F., J. Samaj, A. Hlavacka, J. Kendrick-Jones, and D. Volkmann. 2004. Actin-dependent fluid-phase endocytosis in inner cortex cells of maize root apices. J. Exp. Bot. 55:463-473. - PubMed
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finbert. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. - PubMed
-
- Borras, T. 2003. Recent developments in ocular gene therapy. Exp. Eye Res. 76:643-652. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- EY 13949/EY/NEI NIH HHS/United States
- R01 EY017293/EY/NEI NIH HHS/United States
- R03 EY013949/EY/NEI NIH HHS/United States
- EY 05081/EY/NEI NIH HHS/United States
- DK 34316/DK/NIDDK NIH HHS/United States
- NS 38246/NS/NINDS NIH HHS/United States
- EY 11386/EY/NEI NIH HHS/United States
- R01 NS038246/NS/NINDS NIH HHS/United States
- R01 EY011386/EY/NEI NIH HHS/United States
- R01 DK034316/DK/NIDDK NIH HHS/United States
- R01 CA102126/CA/NCI NIH HHS/United States
- P30 DK048522/DK/NIDDK NIH HHS/United States
- EY 17293/EY/NEI NIH HHS/United States
- P30 DK 48522/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

