Acetyl-coenzyme A carboxylases: versatile targets for drug discovery
- PMID: 16983687
- PMCID: PMC3837461
- DOI: 10.1002/jcb.21077
Acetyl-coenzyme A carboxylases: versatile targets for drug discovery
Abstract
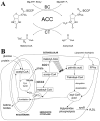
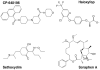
Acetyl-coenzyme A carboxylases (ACCs) have crucial roles in fatty acid metabolism in humans and most other living organisms. They are attractive targets for drug discovery against a variety of human diseases, including diabetes, obesity, cancer, and microbial infections. In addition, ACCs from grasses are the targets of herbicides that have been in commercial use for more than 20 years. Significant progresses in both basic research and in drug discovery have been made over the past few years in the studies on these enzymes. At the basic research level, the crystal structures of the biotin carboxylase (BC) and the carboxyltransferase (CT) components of ACC have been determined, and the molecular basis for ACC inhibition by small molecules are beginning to be understood. At the drug discovery level, a large number of nanomolar inhibitors of mammalian ACCs have been reported and the extent of their therapeutic potential is being aggressively explored. This review summarizes these new progresses and also offers some prospects in terms of the future directions for the studies on these important enzymes.
(c) 2006 Wiley-Liss, Inc.
Figures


Similar articles
-
Targeting acetyl-CoA carboxylases: small molecular inhibitors and their therapeutic potential.Recent Pat Anticancer Drug Discov. 2012 May 1;7(2):168-84. doi: 10.2174/157489212799972918. Recent Pat Anticancer Drug Discov. 2012. PMID: 22339356 Review.
-
Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5910-5. doi: 10.1073/pnas.0400891101. Epub 2004 Apr 12. Proc Natl Acad Sci U S A. 2004. PMID: 15079078 Free PMC article.
-
Chemical genetics of acetyl-CoA carboxylases.Molecules. 2013 Jan 28;18(2):1704-19. doi: 10.3390/molecules18021704. Molecules. 2013. PMID: 23358327 Free PMC article. Review.
-
Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase.Science. 2003 Mar 28;299(5615):2064-7. doi: 10.1126/science.1081366. Science. 2003. PMID: 12663926
-
Structure and function of biotin-dependent carboxylases.Cell Mol Life Sci. 2013 Mar;70(5):863-91. doi: 10.1007/s00018-012-1096-0. Epub 2012 Aug 7. Cell Mol Life Sci. 2013. PMID: 22869039 Free PMC article. Review.
Cited by
-
Trypanosoma brucei: inhibition of acetyl-CoA carboxylase by haloxyfop.Exp Parasitol. 2012 Feb;130(2):159-65. doi: 10.1016/j.exppara.2011.10.014. Epub 2011 Nov 19. Exp Parasitol. 2012. PMID: 22119241 Free PMC article.
-
Recombinant yeast screen for new inhibitors of human acetyl-CoA carboxylase 2 identifies potential drugs to treat obesity.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9093-8. doi: 10.1073/pnas.1003721107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439761 Free PMC article.
-
Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy.Nat Biotechnol. 2008 Oct;26(10):1179-86. doi: 10.1038/nbt.1500. Epub 2008 Sep 28. Nat Biotechnol. 2008. PMID: 18820684 Free PMC article.
-
Human acetyl-CoA carboxylase 2 expressed in silkworm Bombyx mori exhibits posttranslational biotinylation and phosphorylation.Appl Microbiol Biotechnol. 2014 Oct;98(19):8201-9. doi: 10.1007/s00253-014-5715-6. Epub 2014 Apr 17. Appl Microbiol Biotechnol. 2014. PMID: 24740690 Free PMC article.
-
Mining of potential drug targets through the identification of essential and analogous enzymes in the genomes of pathogens of Glycine max, Zea mays and Solanum lycopersicum.PLoS One. 2018 May 25;13(5):e0197511. doi: 10.1371/journal.pone.0197511. eCollection 2018. PLoS One. 2018. PMID: 29799863 Free PMC article.
References
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KAH, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
-
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

