Higher order Rab programming in phagolysosome biogenesis
- PMID: 16982798
- PMCID: PMC2064384
- DOI: 10.1083/jcb.200603026
Higher order Rab programming in phagolysosome biogenesis
Abstract
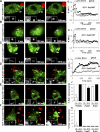
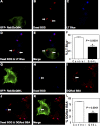
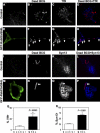
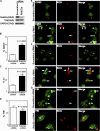
Phagosomes offer kinetically and morphologically tractable organelles to dissect the control of phagolysosome biogenesis by Rab GTPases. Model phagosomes harboring latex beads undergo a coordinated Rab5-Rab7 exchange, which is akin to the process of endosomal Rab conversion, the control mechanisms of which are unknown. In the process of blocking phagosomal maturation, the intracellular pathogen Mycobacterium tuberculosis prevents Rab7 acquisition, thus, providing a naturally occurring tool to study Rab conversion. We show that M. tuberculosis inhibition of Rab7 acquisition and arrest of phagosomal maturation depends on Rab22a. Four-dimensional microscopy revealed that phagosomes harboring live mycobacteria recruited and retained increasing amounts of Rab22a. Rab22a knockdown in macrophages via siRNA enhanced the maturation of phagosomes with live mycobacteria. Conversely, overexpression of the GTP-locked mutant Rab22aQ64L prevented maturation of phagosomes containing heat-killed mycobacteria, which normally progress into phagolysosomes. Moreover, Rab22a knockdown led to Rab7 acquisition by phagosomes harboring live mycobacteria. Our findings show that Rab22a defines the critical checkpoint for Rab7 conversion on phagosomes, allowing or disallowing organellar transition into a late endosomal compartment. M. tuberculosis parasitizes this process by actively recruiting and maintaining Rab22a on its phagosome, thus, preventing Rab7 acquisition and blocking phagolysosomal biogenesis.
Figures

Similar articles
-
Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest.EMBO J. 2006 Nov 15;25(22):5250-9. doi: 10.1038/sj.emboj.7601407. Epub 2006 Nov 2. EMBO J. 2006. PMID: 17082769 Free PMC article.
-
Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes.Traffic. 2011 Apr;12(4):407-20. doi: 10.1111/j.1600-0854.2011.01165.x. Epub 2011 Feb 21. Traffic. 2011. PMID: 21255211
-
Mycobacterium bovis BCG disrupts the interaction of Rab7 with RILP contributing to inhibition of phagosome maturation.J Leukoc Biol. 2007 Dec;82(6):1437-45. doi: 10.1189/jlb.0507289. J Leukoc Biol. 2007. PMID: 18040083
-
[Alterations in recruitment and activation of Rab proteins during mycobacterial infection].Biomedica. 2010 Apr-Jun;30(2):283-308. Biomedica. 2010. PMID: 20890576 Review. Spanish.
-
Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking.Electrophoresis. 1997 Dec;18(14):2542-7. doi: 10.1002/elps.1150181409. Electrophoresis. 1997. PMID: 9527483 Review.
Cited by
-
Interactions between Leishmania braziliensis and Macrophages Are Dependent on the Cytoskeleton and Myosin Va.J Parasitol Res. 2012;2012:275436. doi: 10.1155/2012/275436. Epub 2012 Jun 27. J Parasitol Res. 2012. PMID: 22792440 Free PMC article.
-
From infection niche to therapeutic target: the intracellular lifestyle of Mycobacterium tuberculosis.Microbiology (Reading). 2021 Apr;167(4):001041. doi: 10.1099/mic.0.001041. Microbiology (Reading). 2021. PMID: 33826491 Free PMC article. Review.
-
Mycobacterial infection induces a specific human innate immune response.Sci Rep. 2015 Nov 20;5:16882. doi: 10.1038/srep16882. Sci Rep. 2015. PMID: 26586179 Free PMC article.
-
Differential requirement of Rab22a for the recruitment of ER-derived proteins to phagosomes and endosomes in dendritic cells.Small GTPases. 2020 May;11(3):211-219. doi: 10.1080/21541248.2017.1384088. Epub 2018 Jan 24. Small GTPases. 2020. PMID: 28960134 Free PMC article.
-
The complexity of Rab5 to Rab7 transition guarantees specificity of pathogen subversion mechanisms.Front Cell Infect Microbiol. 2014 Dec 22;4:180. doi: 10.3389/fcimb.2014.00180. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25566515 Free PMC article. Review. No abstract available.
References
-
- Alvarez-Dominguez, C., A.M. Barbieri, W. Beron, A. Wandinger-Ness, and P.D. Stahl. 1996. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834–13843. - PubMed
-
- Chua, J., and V. Deretic. 2004. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J. Biol. Chem. 279:36982–36992. - PubMed

