ORF66 protein kinase function is required for T-cell tropism of varicella-zoster virus in vivo
- PMID: 16971426
- PMCID: PMC1642581
- DOI: 10.1128/JVI.00466-06
ORF66 protein kinase function is required for T-cell tropism of varicella-zoster virus in vivo
Abstract
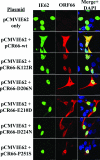
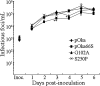
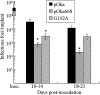
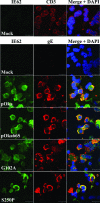
Several functions have been attributed to the serine/threonine protein kinase encoded by open reading frame 66 (ORF66) of varicella-zoster virus (VZV), including modulation of the apoptosis and interferon pathways, down-regulation of major histocompatibility complex class I cell surface expression, and regulation of IE62 localization. The amino acid sequence of the ORF66 protein contains a recognizable conserved kinase domain. Point mutations were introduced into conserved protein kinase motifs to evaluate their importance to ORF66 protein functions. Two substitution mutants were generated, including a G102A substitution, which blocked autophosphorylation and altered IE62 localization, and an S250P substitution, which had no effect on either autophosphorylation or IE62 localization. Both kinase domain mutants grew to titers equivalent to recombinant parent Oka (pOka) in vitro. pOka66G102A had slightly reduced growth in skin, which was comparable to the reduction observed when ORF66 translation was prevented by stop codon insertions in pOka66S. In contrast, infection of T-cell xenografts with pOka66G102A was associated with a significant decrease in infectious virus production equivalent to the impaired T-cell tropism found with pOka66S infection of T-cell xenografts in vivo. Disrupting kinase activity with the G102A mutation did not alter IE62 cytoplasmic localization in VZV-infected T cells, suggesting that decreased T-cell tropism is due to other ORF66 protein functions. The G102A mutation reduced the antiapoptotic effects of VZV infection of T cells. These experiments indicate that the T-cell tropism of VZV depends upon intact ORF66 protein kinase function.
Figures



Similar articles
-
T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection.J Virol. 2005 Oct;79(20):12921-33. doi: 10.1128/JVI.79.20.12921-12933.2005. J Virol. 2005. PMID: 16188994 Free PMC article.
-
Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase.J Virol. 2000 Mar;74(5):2265-77. doi: 10.1128/jvi.74.5.2265-2277.2000. J Virol. 2000. PMID: 10666257 Free PMC article.
-
The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo.J Virol. 2004 Feb;78(3):1181-94. doi: 10.1128/jvi.78.3.1181-1194.2004. J Virol. 2004. PMID: 14722273 Free PMC article.
-
Varicella-zoster virus T cell tropism and the pathogenesis of skin infection.Curr Top Microbiol Immunol. 2010;342:189-209. doi: 10.1007/82_2010_29. Curr Top Microbiol Immunol. 2010. PMID: 20397071 Free PMC article. Review.
-
Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases.Curr Top Microbiol Immunol. 2010;342:79-98. doi: 10.1007/82_2009_7. Curr Top Microbiol Immunol. 2010. PMID: 20186610 Free PMC article. Review.
Cited by
-
Insights From Studies of the Genetics, Pathogenesis, and Immunogenicity of the Varicella Vaccine.J Infect Dis. 2022 Oct 21;226(Suppl 4):S385-S391. doi: 10.1093/infdis/jiac278. J Infect Dis. 2022. PMID: 36265853 Free PMC article.
-
Role for the Ventral Posterior Medial/Posterior Lateral Thalamus and Anterior Cingulate Cortex in Affective/Motivation Pain Induced by Varicella Zoster Virus.Front Integr Neurosci. 2017 Oct 16;11:27. doi: 10.3389/fnint.2017.00027. eCollection 2017. Front Integr Neurosci. 2017. PMID: 29089872 Free PMC article.
-
Infected peripheral blood mononuclear cells transmit latent varicella zoster virus infection to the guinea pig enteric nervous system.J Neurovirol. 2014 Oct;20(5):442-56. doi: 10.1007/s13365-014-0259-1. Epub 2014 Jun 26. J Neurovirol. 2014. PMID: 24965252 Free PMC article.
-
Sex differences underlying orofacial varicella zoster associated pain in rats.BMC Neurol. 2017 May 17;17(1):95. doi: 10.1186/s12883-017-0882-6. BMC Neurol. 2017. PMID: 28514943 Free PMC article.
-
Age and immune status of rhesus macaques impact simian varicella virus gene expression in sensory ganglia.J Virol. 2013 Aug;87(15):8294-306. doi: 10.1128/JVI.01112-13. Epub 2013 May 22. J Virol. 2013. PMID: 23698305 Free PMC article.
References
-
- Arvin, A. 2001.Varicella-zoster virus, p. 2731-2767. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
-
- Baiker, A., C. Bagowski, H. Ito, M. Sommer, L. Zerboni, K. Fabel, J. Hay, W. Ruyechan, and A. M. Arvin. 2004. The immediate-early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCID-hu model in vivo. J. Virol. 78:1181-1194. - PMC - PubMed
-
- Besser, J., M. H. Sommer, L. Zerboni, C. P. Bagowski, H. Ito, J. Moffat, C.-C. Ku, and A. M. Arvin. 2003. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J. Virol. 77:5964-5974. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

