Role of fascin in filopodial protrusion
- PMID: 16966425
- PMCID: PMC2064340
- DOI: 10.1083/jcb.200603013
Role of fascin in filopodial protrusion
Abstract
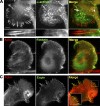
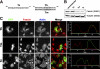
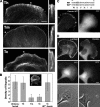
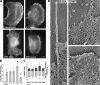
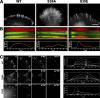
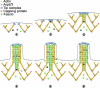
In this study, the mechanisms of actin-bundling in filopodia were examined. Analysis of cellular localization of known actin cross-linking proteins in mouse melanoma B16F1 cells revealed that fascin was specifically localized along the entire length of all filopodia, whereas other actin cross-linkers were not. RNA interference of fascin reduced the number of filopodia, and remaining filopodia had abnormal morphology with wavy and loosely bundled actin organization. Dephosphorylation of serine 39 likely determined cellular filopodia frequency. The constitutively active fascin mutant S39A increased the number and length of filopodia, whereas the inactive fascin mutant S39E reduced filopodia frequency. Fluorescence recovery after photobleaching of GFP-tagged wild-type and S39A fascin showed that dephosphorylated fascin underwent rapid cycles of association to and dissociation from actin filaments in filopodia, with t(1/2) < 10 s. We propose that fascin is a key specific actin cross-linker, providing stiffness for filopodial bundles, and that its dynamic behavior allows for efficient coordination between elongation and bundling of filopodial actin filaments.
Figures



Similar articles
-
Filopodia formation by crosslinking of F-actin with fascin in two different binding manners.Cytoskeleton (Hoboken). 2016 Jun;73(7):365-74. doi: 10.1002/cm.21309. Epub 2016 Jun 13. Cytoskeleton (Hoboken). 2016. PMID: 27169557
-
Intrinsic dynamic behavior of fascin in filopodia.Mol Biol Cell. 2007 Oct;18(10):3928-40. doi: 10.1091/mbc.e07-04-0346. Epub 2007 Aug 1. Mol Biol Cell. 2007. PMID: 17671164 Free PMC article.
-
Fascin in lamellipodia contributes to cell elasticity by controlling the orientation of filamentous actin.Genes Cells. 2019 Mar;24(3):202-213. doi: 10.1111/gtc.12671. Genes Cells. 2019. PMID: 30664308
-
The post-translational modification of Fascin: impact on cell biology and its associations with inhibiting tumor metastasis.Amino Acids. 2022 Dec;54(12):1541-1552. doi: 10.1007/s00726-022-03193-x. Epub 2022 Aug 8. Amino Acids. 2022. PMID: 35939077 Review.
-
How does fascin promote cancer metastasis?FEBS J. 2021 Mar;288(5):1434-1446. doi: 10.1111/febs.15484. Epub 2020 Jul 23. FEBS J. 2021. PMID: 32657526 Free PMC article. Review.
Cited by
-
Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma.BMC Cancer. 2012 Jan 20;12:32. doi: 10.1186/1471-2407-12-32. BMC Cancer. 2012. PMID: 22264292 Free PMC article.
-
Involvement of DNMT 3B promotes epithelial-mesenchymal transition and gene expression profile of invasive head and neck squamous cell carcinomas cell lines.BMC Cancer. 2016 Jul 8;16:431. doi: 10.1186/s12885-016-2468-x. BMC Cancer. 2016. PMID: 27391030 Free PMC article.
-
Contribution of Filopodia to Cell Migration: A Mechanical Link between Protrusion and Contraction.Int J Cell Biol. 2010;2010:507821. doi: 10.1155/2010/507821. Epub 2010 Jul 6. Int J Cell Biol. 2010. PMID: 20671957 Free PMC article.
-
Actin cross-link assembly and disassembly mechanics for alpha-Actinin and fascin.J Biol Chem. 2010 Aug 20;285(34):26350-7. doi: 10.1074/jbc.M110.123117. Epub 2010 Jun 15. J Biol Chem. 2010. PMID: 20551315 Free PMC article.
-
A role for RASSF1A in tunneling nanotube formation between cells through GEFH1/Rab11 pathway control.Cell Commun Signal. 2018 Oct 11;16(1):66. doi: 10.1186/s12964-018-0276-4. Cell Commun Signal. 2018. PMID: 30305100 Free PMC article.
References
-
- Al-Alwan, M.M., G. Rowden, T.D. Lee, and K.A. West. 2001. Fascin is involved in the antigen presentation activity of mature dendritic cells. J. Immunol. 166:338–345. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

