Small molecules that bind the inner core of gp41 and inhibit HIV envelope-mediated fusion
- PMID: 16963566
- PMCID: PMC1599892
- DOI: 10.1073/pnas.0601036103
Small molecules that bind the inner core of gp41 and inhibit HIV envelope-mediated fusion
Abstract
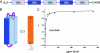
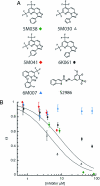
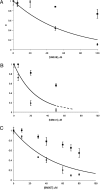
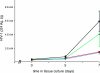
HIV-1 enters cells by membrane fusion, mediated by the trimeric viral envelope glycoprotein gp160, which is processed by a single proteolytic cleavage into stably associated gp120 and gp41. The gp120/gp41 trimer can be triggered to undergo an irreversible conformational change. Using a protein-based assay designed to mimic the gp41 conformational change, we screened for small molecules that prevent the formation of postfusion gp41. Several compounds were identified. One set of structurally related molecules inhibited formation of a postfusion-like assembly with an IC50 of approximately 5 microM. The compounds also inhibited envelope-mediated membrane fusion in both cell-cell fusion and viral infectivity assays. Thus, our screen identifies effective fusion inhibitors. Tested against a panel of envelope proteins from primary HIV-1 isolates, the compounds inhibited fusion across a broad range of clades, including both M and T tropic strains. They bind in a highly conserved, hydrophobic pocket on the inner core of the gp41 trimer, a region previously identified as a potential inhibitor site.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
The Polar Region of the HIV-1 Envelope Protein Determines Viral Fusion and Infectivity by Stabilizing the gp120-gp41 Association.J Virol. 2019 Mar 21;93(7):e02128-18. doi: 10.1128/JVI.02128-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651369 Free PMC article.
-
Functional analysis of the disulfide-bonded loop/chain reversal region of human immunodeficiency virus type 1 gp41 reveals a critical role in gp120-gp41 association.J Virol. 2001 Jul;75(14):6635-44. doi: 10.1128/JVI.75.14.6635-6644.2001. J Virol. 2001. PMID: 11413331 Free PMC article.
-
Conformation of trimeric envelope glycoproteins: the CD4-dependent membrane fusion mechanism of HIV-1.J Biomol Struct Dyn. 2007 Aug;25(1):1-9. doi: 10.1080/07391102.2007.10507150. J Biomol Struct Dyn. 2007. PMID: 17676933
-
Viral surface glycoproteins, gp120 and gp41, as potential drug targets against HIV-1: brief overview one quarter of a century past the approval of zidovudine, the first anti-retroviral drug.Eur J Med Chem. 2011 Apr;46(4):979-92. doi: 10.1016/j.ejmech.2011.01.046. Epub 2011 Feb 3. Eur J Med Chem. 2011. PMID: 21345545 Review.
-
Small-molecule HIV-1 entry inhibitors targeting gp120 and gp41: a patent review (2010-2015).Expert Opin Ther Pat. 2017 Jun;27(6):707-719. doi: 10.1080/13543776.2017.1281249. Epub 2017 Jan 19. Expert Opin Ther Pat. 2017. PMID: 28076686 Review.
Cited by
-
Broad distribution of energetically important contacts across an extended protein interface.J Am Chem Soc. 2011 Jul 6;133(26):10038-41. doi: 10.1021/ja203358t. Epub 2011 Jun 14. J Am Chem Soc. 2011. PMID: 21644542 Free PMC article.
-
A novel view of modelling interactions between synthetic and biological polymers via docking.J Comput Aided Mol Des. 2012 Dec;26(12):1369-88. doi: 10.1007/s10822-012-9621-7. Epub 2012 Dec 13. J Comput Aided Mol Des. 2012. PMID: 23239170
-
Structural and biological mimicry of protein surface recognition by alpha/beta-peptide foldamers.Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14751-6. doi: 10.1073/pnas.0902663106. Epub 2009 Aug 17. Proc Natl Acad Sci U S A. 2009. PMID: 19706443 Free PMC article.
-
Origins of resistance to the HIVgp41 viral entry inhibitor T20.Biochemistry. 2010 May 4;49(17):3575-92. doi: 10.1021/bi901915g. Biochemistry. 2010. PMID: 20230061 Free PMC article.
-
Divide and conquer: high resolution structural information on TRP channel fragments.J Gen Physiol. 2009 Mar;133(3):231-7. doi: 10.1085/jgp.200810137. J Gen Physiol. 2009. PMID: 19237587 Free PMC article. Review. No abstract available.
References
-
- Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. Science. 1985;228:1091–1094. - PubMed
-
- Veronese FD, DeVico AL, Copeland TD, Oroszlan S, Gallo RC, Sarngadharan MG. Science. 1985;229:1402–1405. - PubMed
-
- Gallaher WR. Cell. 1987;50:327–328. - PubMed
-
- Moore JP, McKeating JA, Weiss RA, Sattentau QJ. Science. 1990;250:1139–1142. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

