Unique model of dormant infection for tuberculosis vaccine development
- PMID: 16960113
- PMCID: PMC1563568
- DOI: 10.1128/CVI.00120-06
Unique model of dormant infection for tuberculosis vaccine development
Abstract
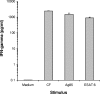
Most individuals exposed to Mycobacterium tuberculosis become infected but hinder the infectious process in dormant foci, known as latent tuberculosis. This limited infection usually stimulates strong T-cell responses, which provide lifelong resistance to tuberculosis. However, latent tuberculosis is still poorly understood, particularly because of the lack of a reliable animal model of dormant infection. Here we show that inoculation of mice with a unique streptomycin-auxotrophic mutant of Mycobacterium tuberculosis recapitulates dormant infection. The mutant grows unimpaired in the presence of streptomycin and no longer grows but remains viable for long periods of time after substrate removal, shifting from the log growth phase to the latent stage, as indicated by augmented production of alpha-crystallin. Mice challenged with the mutant and inoculated with streptomycin for approximately 3 weeks developed a limited infection characterized by a low bacteriological burden and the presence of typical granulomas. After substrate withdrawal, the infection was hindered but few microorganisms remained viable (dormant) in the animals' tissues for at least 6 months. In addition, the animals developed both potent T-cell responses to M. tuberculosis antigens, such as early culture filtrate, Ag85B, and ESAT-6, and resistance to reinfection with virulent M. tuberculosis. Therefore, infection of mice or other animals (e.g., guinea pigs) with M. tuberculosis strain 18b constitutes a simple and attractive animal model for evaluation of antituberculosis vaccines in the context of an M. tuberculosis-presensitized host, a prevailing condition among humans in need of a vaccine.
Figures



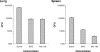
Similar articles
-
Immunogenicity and protective efficacy of a fusion protein vaccine consisting of antigen Ag85B and HspX against Mycobacterium tuberculosis infection in mice.Scand J Immunol. 2011 Jun;73(6):568-76. doi: 10.1111/j.1365-3083.2011.02531.x. Scand J Immunol. 2011. PMID: 21323695
-
Enhanced protection against tuberculosis by vaccination with recombinant Mycobacterium microti vaccine that induces T cell immunity against region of difference 1 antigens.J Infect Dis. 2004 Jul 1;190(1):115-22. doi: 10.1086/421468. Epub 2004 Jun 4. J Infect Dis. 2004. PMID: 15195250
-
A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection.Appl Microbiol Biotechnol. 2015 Dec;99(24):10587-95. doi: 10.1007/s00253-015-6962-x. Epub 2015 Sep 12. Appl Microbiol Biotechnol. 2015. PMID: 26363555
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Animal models of tuberculosis: Guinea pigs.Cold Spring Harb Perspect Med. 2014 Dec 18;5(5):a018572. doi: 10.1101/cshperspect.a018572. Cold Spring Harb Perspect Med. 2014. PMID: 25524720 Free PMC article. Review.
Cited by
-
Mouse Models for Mycobacterium tuberculosis Pathogenesis: Show and Do Not Tell.Pathogens. 2022 Dec 28;12(1):49. doi: 10.3390/pathogens12010049. Pathogens. 2022. PMID: 36678397 Free PMC article. Review.
-
Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish.PLoS Pathog. 2012 Sep;8(9):e1002944. doi: 10.1371/journal.ppat.1002944. Epub 2012 Sep 27. PLoS Pathog. 2012. PMID: 23028333 Free PMC article.
-
Simple model for testing drugs against nonreplicating Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2010 Oct;54(10):4150-8. doi: 10.1128/AAC.00821-10. Epub 2010 Aug 2. Antimicrob Agents Chemother. 2010. PMID: 20679505 Free PMC article.
-
Initiation of Post-Primary Tuberculosis of the Lungs: Exploring the Secret Role of Bone Marrow Derived Stem Cells.Front Immunol. 2021 Jan 21;11:594572. doi: 10.3389/fimmu.2020.594572. eCollection 2020. Front Immunol. 2021. PMID: 33584661 Free PMC article. Review.
-
Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β.Nat Immunol. 2013 Jan;14(1):52-60. doi: 10.1038/ni.2474. Epub 2012 Nov 18. Nat Immunol. 2013. PMID: 23160153 Free PMC article.
References
-
- Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. - PubMed
-
- Cardoso, F. L., P. R. Antas, A. S. Milagres, A. Geluk, K. L. Franken, E. B. Oliveira, H. C. Teixeira, S. A. Nogueira, E. N. Sarno, P. Klatser, T. H. Ottenhoff, and E. P. Sampaio. 2002. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infect. Immun. 70:6707-6714. - PMC - PubMed
-
- Collins, F. M., and V. Montalbine. 1976. Distribution of mycobacteria grown in vivo in the organs of intravenously infected mice. Am. Rev. Respir. Dis. 113:281-286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

