The yeast actin cytoskeleton: from cellular function to biochemical mechanism
- PMID: 16959963
- PMCID: PMC1594590
- DOI: 10.1128/MMBR.00013-06
The yeast actin cytoskeleton: from cellular function to biochemical mechanism
Abstract
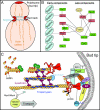
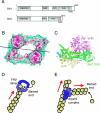
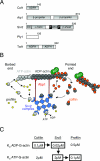
All cells undergo rapid remodeling of their actin networks to regulate such critical processes as endocytosis, cytokinesis, cell polarity, and cell morphogenesis. These events are driven by the coordinated activities of a set of 20 to 30 highly conserved actin-associated proteins, in addition to many cell-specific actin-associated proteins and numerous upstream signaling molecules. The combined activities of these factors control with exquisite precision the spatial and temporal assembly of actin structures and ensure dynamic turnover of actin structures such that cells can rapidly alter their cytoskeletons in response to internal and external cues. One of the most exciting principles to emerge from the last decade of research on actin is that the assembly of architecturally diverse actin structures is governed by highly conserved machinery and mechanisms. With this realization, it has become apparent that pioneering efforts in budding yeast have contributed substantially to defining the universal mechanisms regulating actin dynamics in eukaryotes. In this review, we first describe the filamentous actin structures found in Saccharomyces cerevisiae (patches, cables, and rings) and their physiological functions, and then we discuss in detail the specific roles of actin-associated proteins and their biochemical mechanisms of action.
Figures

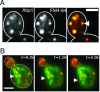
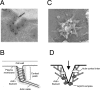
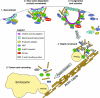
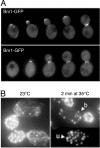





Similar articles
-
The yeast actin cytoskeleton.FEMS Microbiol Rev. 2014 Mar;38(2):213-27. doi: 10.1111/1574-6976.12064. Epub 2014 Feb 20. FEMS Microbiol Rev. 2014. PMID: 24467403 Review.
-
Role of formins in actin assembly: nucleation and barbed-end association.Science. 2002 Jul 26;297(5581):612-5. doi: 10.1126/science.1072309. Epub 2002 Jun 6. Science. 2002. PMID: 12052901
-
Yeast formins regulate cell polarity by controlling the assembly of actin cables.Nat Cell Biol. 2002 Jan;4(1):42-50. doi: 10.1038/ncb719. Nat Cell Biol. 2002. PMID: 11740491
-
Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton.J Cell Biol. 1997 Mar 24;136(6):1165-8. doi: 10.1083/jcb.136.6.1165. J Cell Biol. 1997. PMID: 9087434 Free PMC article. Review. No abstract available.
-
Yeast actin patches are networks of branched actin filaments.J Cell Biol. 2004 Aug 30;166(5):629-35. doi: 10.1083/jcb.200404159. J Cell Biol. 2004. PMID: 15337772 Free PMC article.
Cited by
-
Internetwork competition for monomers governs actin cytoskeleton organization.Nat Rev Mol Cell Biol. 2016 Dec;17(12):799-810. doi: 10.1038/nrm.2016.106. Epub 2016 Sep 14. Nat Rev Mol Cell Biol. 2016. PMID: 27625321 Free PMC article.
-
Dynamics of Actin Cables in Polarized Growth of the Filamentous Fungus Aspergillus nidulans.Front Microbiol. 2016 May 9;7:682. doi: 10.3389/fmicb.2016.00682. eCollection 2016. Front Microbiol. 2016. PMID: 27242709 Free PMC article.
-
Actin and endocytosis in budding yeast.Genetics. 2015 Feb;199(2):315-58. doi: 10.1534/genetics.112.145540. Genetics. 2015. PMID: 25657349 Free PMC article. Review.
-
Cytoskeletal impairment during isoamyl alcohol-induced cell elongation in budding yeast.Sci Rep. 2016 Aug 10;6:31127. doi: 10.1038/srep31127. Sci Rep. 2016. PMID: 27507042 Free PMC article.
-
Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly.Chem Biol. 2009 Nov 25;16(11):1158-68. doi: 10.1016/j.chembiol.2009.10.006. Chem Biol. 2009. PMID: 19942139 Free PMC article.
References
-
- Adams, A. E., D. Botstein, and D. G. Drubin. 1991. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature 354:404-408. - PubMed
-
- Adams, A. E., D. Botstein, and D. G. Drubin. 1989. A yeast actin-binding protein is encoded by SAC6, a gene found by suppression of an actin mutation. Science 243:231-233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

