Delayed Nogo receptor therapy improves recovery from spinal cord contusion
- PMID: 16958113
- PMCID: PMC2855693
- DOI: 10.1002/ana.20953
Delayed Nogo receptor therapy improves recovery from spinal cord contusion
Abstract
Objective: Myelin-associated inhibitors play a role in limiting axonal growth in the adult central nervous system. Blocking these inhibitors may promote neurological recovery from spinal cord contusion.
Methods: The soluble Nogo-66 receptor (NgR(310)ecto-Fc) protein, which can neutralize three myelin inhibitors, was infused into rats after spinal cord contusion for 28 days. Treatment was initiated intrathecally at the time of injury or 3 days after injury by the intracerebroventricular route at a dose of 0.29 mg/kg/day. Recovery of locomotion and of axonal growth was assessed. Some animals received combination therapy with NgR(310)ecto-Fc plus rolipram, a cyclic adenosine monophosphate phosphodiesterase inhibitor.
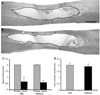
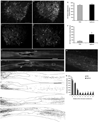
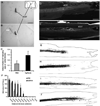
Results: Seven weeks after spinal injury, the Basso-Beattie Bresnahan locomotor scores were significantly improved in the 3-day delayed NgR(310)ecto-Fc treatment group (9.5 +/- 0.7; n = 16) versus the vehicle-treated group, (6.75 +/- 0.7; n = 15) (p < or = 0.01, analysis of variance). The percentage of NgR(310)ecto-Fc-treated animals able to support their weight was twice that in the control group. Delayed therapy was as efficacious as acute therapy. Addition of rolipram did not alter recovery. The beneficial behavioral effects of NgR(310)ecto-Fc correlated with sprouting of raphespinal axons in the caudal spinal cord and of corticospinal axons in the rostral spinal cord.
Interpretation: NgR(310)ecto-Fc treatment improves outcome in a rodent model that closely mimicked human spinal cord injury.
Figures


Similar articles
-
Effect of combined treatment with methylprednisolone and soluble Nogo-66 receptor after rat spinal cord injury.Eur J Neurosci. 2005 Aug;22(3):587-94. doi: 10.1111/j.1460-9568.2005.04241.x. Eur J Neurosci. 2005. PMID: 16101740 Free PMC article.
-
Recovery from chronic spinal cord contusion after Nogo receptor intervention.Ann Neurol. 2011 Nov;70(5):805-21. doi: 10.1002/ana.22527. Ann Neurol. 2011. PMID: 22162062 Free PMC article.
-
Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury.J Neurosci. 2004 Nov 17;24(46):10511-20. doi: 10.1523/JNEUROSCI.2828-04.2004. J Neurosci. 2004. PMID: 15548666 Free PMC article.
-
Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury.Brain. 2020 Jun 1;143(6):1697-1713. doi: 10.1093/brain/awaa116. Brain. 2020. PMID: 32375169 Free PMC article.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
Cited by
-
Nogo receptor deletion and multimodal exercise improve distinct aspects of recovery in cervical spinal cord injury.J Neurotrauma. 2010 Nov;27(11):2055-66. doi: 10.1089/neu.2010.1491. J Neurotrauma. 2010. PMID: 20809785 Free PMC article.
-
The reticulons: a family of proteins with diverse functions.Genome Biol. 2007;8(12):234. doi: 10.1186/gb-2007-8-12-234. Genome Biol. 2007. PMID: 18177508 Free PMC article. Review.
-
The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors.J Neurosci. 2007 Jul 4;27(27):7117-24. doi: 10.1523/JNEUROSCI.1541-07.2007. J Neurosci. 2007. PMID: 17611264 Free PMC article.
-
Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat.Neurorehabil Neural Repair. 2008 May-Jun;22(3):262-78. doi: 10.1177/1545968307308550. Epub 2007 Nov 30. Neurorehabil Neural Repair. 2008. PMID: 18056009 Free PMC article.
-
Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems.Restor Neurol Neurosci. 2008;26(2-3):97-115. Restor Neurol Neurosci. 2008. PMID: 18820405 Free PMC article. Review.
References
-
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. - PubMed
-
- Lee DH, Strittmatter SM, Sah DW. Targeting the Nogo receptor to treat central nervous system injuries. Nat Rev Drug Discov. 2003;2:872–878. - PubMed
-
- Grandpre T, Strittmatter SM. Nogo: a molecular determinant of axonal growth and regeneration. Neuroscientist. 2001;7:377–386. - PubMed
-
- Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. - PubMed
-
- Wang KC, Koprivica V, Kim JA, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

