In vitro and in vivo pharmacology of synthetic olivetol- or resorcinol-derived cannabinoid receptor ligands
- PMID: 16953186
- PMCID: PMC1978428
- DOI: 10.1038/sj.bjp.0706888
In vitro and in vivo pharmacology of synthetic olivetol- or resorcinol-derived cannabinoid receptor ligands
Abstract
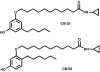
Background and purpose: We have previously reported the development of CB-25 and CB-52, two ligands of CB1 and CB2 cannabinoid receptors. We assessed here their functional activity.
Experimental approach: The effect of the two compounds on forskolin-induced cAMP formation in intact cells or GTP-gamma-S binding to cell membranes, and their action on nociception in vivo was determined.
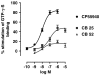
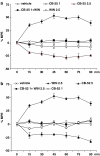
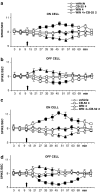
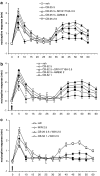
Key results: CB-25 enhanced forskolin-induced cAMP formation in N18TG2 cells (EC50 approximately 20 nM, max. stimulation = 48%), behaving as an inverse CB1 agonist, but it stimulated GTP-gamma-S binding to mouse brain membranes, behaving as a partial CB1 agonist (EC50 =100 nM, max. stimulation = 48%). At human CB1 receptors, CB-25 inhibited cAMP formation in hCB1-CHO cells (EC50 = 1600 nM, max. inhibition = 68% of CP-55,940 effect). CB-52 inhibited forskolin-induced cAMP formation by N18TG2 cells (IC50 = 450 nM, max. inhibition = 40%) and hCB1-CHO cells (EC50 = 2600 nM, max. inhibition = 62% of CP-55,940 effect), and stimulated GTP-gamma-S binding to mouse brain membranes (EC50 = 11 nM, max. stimulation approximately 16%). Both CB-25 and CB-52 showed no activity in all assays of CB2-coupled functional activity and antagonized CP55940-induced stimulation of GTP-gamma-S binding to hCB2-CHO cell membranes. In vivo, both compounds, administered i.p., produced dose-dependent nociception in the plantar test carried out in healthy rats, and antagonised the anti-nociceptive effect of i.p. WIN55,212-2. In the formalin test in mice, however, the compounds counteracted both phases of formalin-induced nociception.
Conclusions and implications: CB-25 and CB-52 behave in vitro mostly as CB1 partial agonists and CB2 neutral antagonists, whereas their activity in vivo might depend on the tonic activity of cannabinoid receptors.
Figures

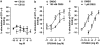
Similar articles
-
Pharmacological characterization of a novel cannabinoid ligand, MDA19, for treatment of neuropathic pain.Anesth Analg. 2010 Jul;111(1):99-109. doi: 10.1213/ANE.0b013e3181e0cdaf. Epub 2010 Jun 3. Anesth Analg. 2010. PMID: 20522703 Free PMC article.
-
Characterization of cannabinoid receptor ligands in tissues natively expressing cannabinoid CB2 receptors.Br J Pharmacol. 2013 Jun;169(4):887-99. doi: 10.1111/bph.12191. Br J Pharmacol. 2013. PMID: 23711022 Free PMC article.
-
Characterization of the pharmacology of imidazolidinedione derivatives at cannabinoid CB1 and CB2 receptors.Eur J Pharmacol. 2004 Jul 8;495(1):43-53. doi: 10.1016/j.ejphar.2004.05.023. Eur J Pharmacol. 2004. PMID: 15219819
-
Cannabinoid receptors and their ligands: ligand-ligand and ligand-receptor modeling approaches.Handb Exp Pharmacol. 2005;(168):247-81. Handb Exp Pharmacol. 2005. PMID: 16596777 Review.
-
CB1 and CB2 cannabinoid receptor binding studies based on modeling and mutagenesis approaches.Mini Rev Med Chem. 2005 Jul;5(7):651-8. doi: 10.2174/1389557054368754. Mini Rev Med Chem. 2005. PMID: 16026311 Review.
Cited by
-
HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications.Curr Chem Genomics. 2009 May 28;3:22-32. doi: 10.2174/1875397300903010022. Curr Chem Genomics. 2009. PMID: 20161833 Free PMC article.
-
Pharmacological characterization of a novel cannabinoid ligand, MDA19, for treatment of neuropathic pain.Anesth Analg. 2010 Jul;111(1):99-109. doi: 10.1213/ANE.0b013e3181e0cdaf. Epub 2010 Jun 3. Anesth Analg. 2010. PMID: 20522703 Free PMC article.
-
Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers.Br J Pharmacol. 2007 Aug;151(7):1061-70. doi: 10.1038/sj.bjp.0707303. Epub 2007 Jun 4. Br J Pharmacol. 2007. PMID: 17549048 Free PMC article.
-
Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity.PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. Epub 2011 Jul 6. PLoS One. 2011. PMID: 21755008 Free PMC article.
-
Cytotoxic Effects of Cannabinoids on Human HT-29 Colorectal Adenocarcinoma Cells: Different Mechanisms of THC, CBD, and CB83.Int J Mol Sci. 2020 Aug 1;21(15):5533. doi: 10.3390/ijms21155533. Int J Mol Sci. 2020. PMID: 32752303 Free PMC article.
References
-
- Beaulieu P, Bisogno T, Punwar S, Farquhar-Smith WP, Ambrosino G, Di Marzo V, et al. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur J Pharmacol. 2000;396:85–92. - PubMed
-
- Brizzi A, Brizzi V, Cascio MG, Bisogno T, Sirianni R, Di Marzo V. Design, synthesis, and binding studies of new potent ligands of cannabinoid receptors. J Med Chem. 2005;48:7343–7350. - PubMed
-
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

